��Ŀ����
����ͭ�ж�����;��������������ɫ��������������ȣ�Ϊ��ô���������ͭ��̽�������ʣ�ijͬѧ�������ܶȻ����ݲ�ͨ������õ��й���Ϣ����������ù�ҵ����ͭ�����������������ʣ���������ʵ�飺
���Ʊ�����ͭ
��ҵCuSO4
CuSO4��Һ
CuSO4?5H2O��CuO
��1�������IJ����Ǽ���ˮ�����������ܽ���Ʒ�����ˣ�Ŀ���dz�ȥ���������ʣ���һ�����м����������
��2�������IJ����ǣ��μ�H2O2��Һ���Լ��ȣ�����Ӧ��ȫ����������Cu2��OH��2CO3��ĩ�����裬�Կ�����ҺpH=3.5���������һ��ʱ�䣬���ˣ���ϡ�����ữ��Һ��pH=1��
����һ�����Ŀ����
��д������H2O2��Һʱ������Ӧ�����ӷ���ʽ
�ۿ�����ҺpH=3.5��Ŀ����
��3��������Ŀ���ǵõ�CuSO4?5H2O���壬������
��̽������ͭ����
��1��ȡA��B��֧�Թܣ���A���ȼ�������CuO��ĩ���ٷֱ���A��B�м���������3%H2O2��Һ��ֻ�۲쵽A���д������ݣ�������
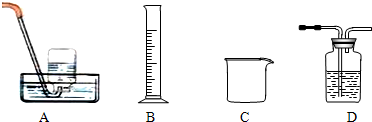
��2��Ϊ̽���Թ�A�з�Ӧ�����ʣ��ռ����岢�ⶨ����������ʵ��������װ��Ϊ
| �� �� | Fe��OH��2 | Cu��OH��2 | Fe��OH��3 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
��ҵCuSO4
| �� |
| �� |
| �� |
��1�������IJ����Ǽ���ˮ�����������ܽ���Ʒ�����ˣ�Ŀ���dz�ȥ���������ʣ���һ�����м����������
��ֹCuSO4������ˮ�⣬����������Ʒ���ܽ�
��ֹCuSO4������ˮ�⣬����������Ʒ���ܽ�
����2�������IJ����ǣ��μ�H2O2��Һ���Լ��ȣ�����Ӧ��ȫ����������Cu2��OH��2CO3��ĩ�����裬�Կ�����ҺpH=3.5���������һ��ʱ�䣬���ˣ���ϡ�����ữ��Һ��pH=1��
����һ�����Ŀ����
��Fe2+ת��ΪFe3+����ȥ
��Fe2+ת��ΪFe3+����ȥ
����д������H2O2��Һʱ������Ӧ�����ӷ���ʽ
2Fe2++H2O2+2H+=2Fe3++2H2O
2Fe2++H2O2+2H+=2Fe3++2H2O
�ۿ�����ҺpH=3.5��Ŀ����
ʹFe3+������ȫ��������Cu2+����������
ʹFe3+������ȫ��������Cu2+����������
����3��������Ŀ���ǵõ�CuSO4?5H2O���壬������
�����ᾧ����
�����ᾧ����
��ˮԡ���Ⱥ�����ù��壮ˮԡ���ȵ��ص��������º͡����Ⱦ��ȡ�����²�����100��
�����º͡����Ⱦ��ȡ�����²�����100��
����̽������ͭ����
��1��ȡA��B��֧�Թܣ���A���ȼ�������CuO��ĩ���ٷֱ���A��B�м���������3%H2O2��Һ��ֻ�۲쵽A���д������ݣ�������
CuO��H2O2�ֽ����˴�������
CuO��H2O2�ֽ����˴�������
����2��Ϊ̽���Թ�A�з�Ӧ�����ʣ��ռ����岢�ⶨ����������ʵ��������װ��Ϊ
BD
BD
������д��ţ�
��������һ��
��1�����ù�ҵ����ͭ�Ʊ�����ͭ���������е��Ʊ����̣��������Ҫ���ܽ���Ʒ����ȥ���������ʣ��õ���������������������ͭ��Һ��ͭ���ӡ��������Ӷ������������ӣ�ˮ��Һ����ˮ�⣬���Լ��������������ˮ�⣬ʹ��Ʒ���õ��ܽ⣻
��2���μ�H2O2��Һ���Լ��ȣ�Ŀ����������������Ϊ���������ӱ��ڳ�ȥ���Ҳ��������ʣ�����Ӧ��ȫ����������Cu2��OH��2CO3��ĩ�����裬�Կ�����ҺpH=3.5������������ӳ�����PH��Χ��֪��Ŀ������������������ȫ������ͭ���Ӳ�������
��3��������Ŀ���ǵõ�CuSO4?5H2O���壬����ͭ��Һ�õ����ʾ���ķ����Ǽ��������ᾧ���ˣ�ˮԡ���ȵ�Ӧ�������ǣ��¶Ȳ�����100��C���軺�����ȼ��ȵĹ��̿�����ˮԡ���ȣ�
������
��1��ȡA��B��֧�Թܣ���A���ȼ�������CuO��ĩ���ٷֱ���A��B�м���������3%H2O2��Һ��ֻ�۲쵽A���д�������˵������ͭ�Թ�������ķֽ����˴����ã�
��2���ռ����岢�ⶨ�������Ҫ�õ����õķ�������ˮ�������������������ѹǿ��ˮѹ����Ͳ���ų�ˮ�������Ϊ�ռ�����������
��1�����ù�ҵ����ͭ�Ʊ�����ͭ���������е��Ʊ����̣��������Ҫ���ܽ���Ʒ����ȥ���������ʣ��õ���������������������ͭ��Һ��ͭ���ӡ��������Ӷ������������ӣ�ˮ��Һ����ˮ�⣬���Լ��������������ˮ�⣬ʹ��Ʒ���õ��ܽ⣻
��2���μ�H2O2��Һ���Լ��ȣ�Ŀ����������������Ϊ���������ӱ��ڳ�ȥ���Ҳ��������ʣ�����Ӧ��ȫ����������Cu2��OH��2CO3��ĩ�����裬�Կ�����ҺpH=3.5������������ӳ�����PH��Χ��֪��Ŀ������������������ȫ������ͭ���Ӳ�������
��3��������Ŀ���ǵõ�CuSO4?5H2O���壬����ͭ��Һ�õ����ʾ���ķ����Ǽ��������ᾧ���ˣ�ˮԡ���ȵ�Ӧ�������ǣ��¶Ȳ�����100��C���軺�����ȼ��ȵĹ��̿�����ˮԡ���ȣ�
������
��1��ȡA��B��֧�Թܣ���A���ȼ�������CuO��ĩ���ٷֱ���A��B�м���������3%H2O2��Һ��ֻ�۲쵽A���д�������˵������ͭ�Թ�������ķֽ����˴����ã�
��2���ռ����岢�ⶨ�������Ҫ�õ����õķ�������ˮ�������������������ѹǿ��ˮѹ����Ͳ���ų�ˮ�������Ϊ�ռ�����������
����⣺��һ����1��ͭ���ӡ����������ܽ�ʱ����ˮ�⣬������Ϊ������ˮ�⣬�ٽ��ܽ⣬�ʴ�Ϊ����ֹCuSO4������ˮ�⣬����������Ʒ���ܽ⣻
��2����ȥ�������ӵõ�����������ͭ��Һ�����ݳ���PH��Χ��֪��Ӧ�Ȱ���������ת��Ϊ���������ӣ��ٿ���PHʹ֮��ȫ���������Եμ�H2O2��ҺĿ����������������Ϊ���������ӣ���������Cu2��OH��2CO3��ĩ�����裬�Կ�����ҺpH=3.5��Ŀ������pH=3.5ʱͭ���Ӳ����������������ӳ�����ȫ���ʴ�Ϊ����Fe2+ת��ΪFe3+����ȥ��ʹFe3+������ȫ��������Cu2+������������
��3����������ͭ��Һ����������ͭ���壬���˵õ����壻ˮԡ������Ϊ�˼��Ⱦ��ȡ��������¶�������100��C���£��ʴ�Ϊ�������ᾧ���� �����º͡����Ⱦ��ȡ�����²�����100�棻
��������1��ȡA��B��֧�Թܣ���A���ȼ�������CuO��ĩ���ٷֱ���A��B�м���������3%H2O2��Һ��ֻ�۲쵽A���д������ݣ�˵����������ֽ�����������ˮ������ͭ�Թ�������ֽ����˴����ã�
�ʴ�Ϊ��CuO��H2O2�ֽ����˴������ã�
��2����ˮ����װ��Ҫ������������ˮ���������������ѹǿ��ˮ�ų�����Ͳ�У�ֱ�Ӷ���ˮ�������Ϊ�ռ�������������ʴ�ѡ��BD��
��2����ȥ�������ӵõ�����������ͭ��Һ�����ݳ���PH��Χ��֪��Ӧ�Ȱ���������ת��Ϊ���������ӣ��ٿ���PHʹ֮��ȫ���������Եμ�H2O2��ҺĿ����������������Ϊ���������ӣ���������Cu2��OH��2CO3��ĩ�����裬�Կ�����ҺpH=3.5��Ŀ������pH=3.5ʱͭ���Ӳ����������������ӳ�����ȫ���ʴ�Ϊ����Fe2+ת��ΪFe3+����ȥ��ʹFe3+������ȫ��������Cu2+������������
��3����������ͭ��Һ����������ͭ���壬���˵õ����壻ˮԡ������Ϊ�˼��Ⱦ��ȡ��������¶�������100��C���£��ʴ�Ϊ�������ᾧ���� �����º͡����Ⱦ��ȡ�����²�����100�棻
��������1��ȡA��B��֧�Թܣ���A���ȼ�������CuO��ĩ���ٷֱ���A��B�м���������3%H2O2��Һ��ֻ�۲쵽A���д������ݣ�˵����������ֽ�����������ˮ������ͭ�Թ�������ֽ����˴����ã�
�ʴ�Ϊ��CuO��H2O2�ֽ����˴������ã�
��2����ˮ����װ��Ҫ������������ˮ���������������ѹǿ��ˮ�ų�����Ͳ�У�ֱ�Ӷ���ˮ�������Ϊ�ռ�������������ʴ�ѡ��BD��
���������⿼����ͭ�Ļ���������ʡ��Ʊ����ᴿ���ӣ����������Ϣ��Ӧ�����������ڲ�ͬPH��Һ�г����ij̶Ȳ�ͬ���г��ӷ���IJ����ԭ����ͬʱ������ʵ�����������

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д� ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ
����ͭ�ж�����;��������������ɫ��������������ȡ�Ϊ��ô���������ͭ��ijͬѧ�ù�ҵ����ͭ(����������������)��������ʵ�飺
���������ϵ�֪������Һ��ͨ��������Һ������Զ�ʹCu2+��Fe2+��Fe3+�ֱ����ɳ�����pH���£�
| ���� | Cu(OH)2 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����pH | 6.0 | 7.5 | 1.4 |
| ������ȫpH | 13 | 14 | 3.7 |
�� ������Ŀ���dz����������������������ȵμ�H2O2��Һ���Լ��ȣ���Fe2+ת����ȫ����������Cu2(OH)2CO3��ĩ�����裬�Ե�����ҺpH��һ����Χ֮�ڣ��������һ��ʱ�䣬���ˣ���ϡ�����ữ��Һ��pH=1��
��д����H2O2��Һ��ȥ�������������ӷ���ʽ____________________________��
�ڵ���pH �ķ�ΧӦ����____________֮�䡣
�� ������Ŀ���ǵõ�CuSO4��5H2O���塣�����ǽ���Һ�����������о�Ĥ����ʱ��ֹͣ���ȣ�_____________��ˮԡ���Ⱥ�ɡ�����ˮԡ���ȵ�ԭ����____ ��
�� ��ͬѧ��CuSO4��Һ��������̽��ʵ�飺ȡA��B��֧�Թܣ��ֱ���� 2 mL 5%H2O2��Һ������H2O2��Һ�зֱ����0.1 mol��L���� FeCl3��CuSO4��Һ��1 mL��ҡ�ȣ��۲쵽����FeCl3 ��Һ���Թܲ������ݸ��죬�ɴ˵õ����ۣ�Fe3+��H2O2��Һ�ֽ�Ĵ�Ч�ʱ�Cu2+�ߣ���ͬѧ�Ľ����Ƿ���ȷ________������ȷ�������˵��ԭ�� ��
��5��16������ͭ����Ͷ��ˮ���γ�1����Һ�������Һ�� �ԣ�����ԡ����ԡ������ԡ�������Һ���������������� 0.1NA������ڡ����ڡ���С�ڡ���������������������Һ����ϡ�����ټ�����Ƭ�����������ʻ����Լӿ죬ԭ���� �������¡�����������������Һ�м�����������ϡ��Һ��ֽ�����dz��ɫ������ͭ�������ɣ�����Һ��pH=8ʱc��Cu2+��=________________mol��L-1��Kap[Cu��OH��2]=2.2��10-20����