��Ŀ����
����Ŀ����һ��������ͷ����Ԫ�ص�����һ�ַ����ǽ����ͷ����ˮ�У�Ƭ�̺�ȡ������Һ���Թ��У���AgNO3��Һ��ϡ�����NaNO2��Һ�������ְ�ɫ������˵������Ԫ�ء�д����������ԭ���������ӷ���ʽ��___________________________��
��������(N2H4)��Ϊһ����Ҫ�ĵ��⻯��������������ߴ�12.5%����ȫ�ֽ����ΪH2��N2����һ�������Һ����Դ��N2H4�ֽ�����з�����ȫ�ֽ�Ͳ���ȫ�ֽ⡣
��ȫ�ֽ⣺ N2H4(g) ![]() N2(g) + 2H2(g) ��H1 = -50.6 kJ��mol-1 I
N2(g) + 2H2(g) ��H1 = -50.6 kJ��mol-1 I
����ȫ�ֽ⣺ 3N2H4(g) ![]() 4NH3(g) + N2(g) ��H2 II
4NH3(g) + N2(g) ��H2 II
��ӦII���ʱ䲻�ײ������ֲ�����������ݣ�
2NH3(g) ![]() N2(g) + 3H2(g) ��H3 = +92 kJ��mol-1 (�÷�Ӧʵ��δ����)
N2(g) + 3H2(g) ��H3 = +92 kJ��mol-1 (�÷�Ӧʵ��δ����)
��1����ӦII��__________(����¡����»������¶�)�����¿����Է����С�
��2��������̶����ܱ������У���Ir/Al2O3Ϊ�������ڲ�ͬ�¶���(������δʧ��)�ֽ����N2H4(һ��ʼ��ϵ������������)����÷�Ӧ��ͬʱ����N2H4��NH3��H2���������ͼ1��ʾ��

������˵����ȷ����_____________
A.��ͼ��֪200��ʱ����Ӧ I�Ļ�ܵ��ڷ�ӦII�Ļ��
B.300���N2H4���������������ߣ��������ڷ�ӦII�����ƶ�����
C.����ѡ����ߵ�Ni/Ir���ϴ��������N2H4��ƽ��ת����
D.400���Ǹ�����ձȽϺ��ʵ������¶�
����ij�¶�����1 L����̶����ܱ������м���1molN2H4����Ӧһ��ʱ���ﵽƽ��״̬����ʱH2��NH3�����ʵ�����Ϊ0.4mol���������¶��·�Ӧ I��ƽ�ⳣ��KC=___________________��KcΪ�������Ũ�ȱ�ʾ��ƽ�ⳣ����
������600~1000��C�½�������ʵ�飬��Ԥ�Ⲣ����ͼ�в���H2��������ı仯����_______��
��3�����ڣ�������λ����Ů��������һ̨����Һ�����ķ��������ԭ���ǰ���Һ�Ե��ķ�ʽ������ṩ�豸����ʹ�õ������ļ�����ͬʱ����Һ�ѵ���ת��Ϊ����ѭ��������Ⱦ���壩����������ˮ�������Դ���ҺΪ���Һ����NH4+��ʾ��Һ�е�Ԫ�صĴ�����ʽ����д���õ����̵��ܷ�Ӧ����ʽ��________________________________________
���𰸡� Ag++3NO2-+ClO3-=AgCl��+3NO3- �����¶� B 0.096 
![]()
����������һ�����ͷ��������أ���NaNO2��Һ��Ӧ��ClO3-����ԭΪCl-��������������Cl-��Ag+��Ӧ�����Ȼ�����ɫ������֤������Ԫ�ء�
��������1����A. ����ͼ���֪����200��ʱ���ȷ�����ӦII��˵����ӦII�Ļ�ܽϵͣ�B.��ӦIIΪ���ȷ�Ӧ�������¶ȣ�ƽ�����ƣ������˵������������������N2H4(g) ![]() N2(g) + 2H2(g) ��H1 =-50.6 kJ��mol-1 ��3N2H4(g)
N2(g) + 2H2(g) ��H1 =-50.6 kJ��mol-1 ��3N2H4(g) ![]() 4NH3(g) + N2(g)��Ӧ��˵������������Ũ�ȣ�ƽ�����ƣ�N2H4���������������ߣ�C. �����ܹ��ӿ췴Ӧ���ʣ�����ƽ�ⲻ�ƶ���D.����ͼ���֪�¶�500������ʱ�������İٷֺ������
4NH3(g) + N2(g)��Ӧ��˵������������Ũ�ȣ�ƽ�����ƣ�N2H4���������������ߣ�C. �����ܹ��ӿ췴Ӧ���ʣ�����ƽ�ⲻ�ƶ���D.����ͼ���֪�¶�500������ʱ�������İٷֺ������
�ڸ��ݷ�ӦN2H4(g) ![]() N2(g) + 2H2(g)
N2(g) + 2H2(g)
��ʼ�� 1 0 0
�仯�� X X 2X
ƽ���� 1-X X 2X
���ݷ�Ӧ 3N2H4(g) ![]() 4NH3(g) + N2(g)
4NH3(g) + N2(g)
��ʼ�� 1-X 0 2X
�仯�� 0.3 0.4 0.1
ƽ���� 1-X-0.3 0.4 0.1+X
������֪��Ϣ�������ӦI��ƽ��Ũ�ȣ�����ƽ�ⳣ����ʽ���м��㡣
�۶���N2H4(g) ![]() N2(g) + 2H2(g) ��H1 = -50.6 kJ��mol-1 ��Ӧ�������¶ȣ�ƽ�����ƣ�H2��������ļ�С�����˹��ɻ�ͼ��
N2(g) + 2H2(g) ��H1 = -50.6 kJ��mol-1 ��Ӧ�������¶ȣ�ƽ�����ƣ�H2��������ļ�С�����˹��ɻ�ͼ��
��3����⺬��NH4+����Һ��-3�۵����ߵ�0�ۣ���Ԫ����+1�۽��͵�0�ۣ��ݴ�д�������̵��ܷ�Ӧ����ʽ��
��һ�����ͷ�к�������أ������ͷ����ˮ�У�Ƭ�̺�ȡ������Һ���Թ��У���AgNO3��Һ��ϡ�����NaNO2��Һ��ClO3-����ԭΪCl-��Cl-��Ag+��Ӧ�����Ȼ�����ɫ������˵������Ԫ�ء����ӷ���ʽ��Ag++3NO2-+ClO3-=AgCl��+3NO3-����ȷ�𰸣�Ag++3NO2-+ClO3-=AgCl��+3NO3-��
��������1�����ݸ�˹���ɣ���ӦI��3-��ӦIII��2�������÷�ӦII��2NH3(g) ![]() N2(g) + 3H2(g) ��H2 = -345.8 kJ��mol-1��S>0����H<0���÷�Ӧ�������¶������¿����Է����У���ȷ�𰸣������¶ȡ�
N2(g) + 3H2(g) ��H2 = -345.8 kJ��mol-1��S>0����H<0���÷�Ӧ�������¶������¿����Է����У���ȷ�𰸣������¶ȡ�
��2���ٸ���ͼ���֪����Ӧ��200��ʱ���������������Ϊ0����������������ϴ�˵����ӦII��������ܱȷ�ӦI�ĵͣ�A����ӦII��2NH3(g) ![]() N2(g) + 3H2(g) ��H3 = +92 kJ��mol-1�������¶ȣ�ƽ�����ƣ�����������С�������������������࣬���N2H4(g)
N2(g) + 3H2(g) ��H3 = +92 kJ��mol-1�������¶ȣ�ƽ�����ƣ�����������С�������������������࣬���N2H4(g) ![]() N2(g) + 2H2(g) ��H1 =-50.6 kJ��mol-1 ��3N2H4(g)
N2(g) + 2H2(g) ��H1 =-50.6 kJ��mol-1 ��3N2H4(g) ![]() 4NH3(g) + N2(g)��Ӧ������������Ũ�ȣ�ƽ�����ƣ�N2H4���������������ߣ�B��ȷ�������ܹ��ӿ췴Ӧ���ʣ�����ƽ�ⲻ�ƶ����������N2H4��ƽ��ת���ʣ�C������ͼ���֪���¶���500������ʱ�������İٷֺ������D������ȷѡ��B��
4NH3(g) + N2(g)��Ӧ������������Ũ�ȣ�ƽ�����ƣ�N2H4���������������ߣ�B��ȷ�������ܹ��ӿ췴Ӧ���ʣ�����ƽ�ⲻ�ƶ����������N2H4��ƽ��ת���ʣ�C������ͼ���֪���¶���500������ʱ�������İٷֺ������D������ȷѡ��B��
�ڸ��ݷ�ӦN2H4(g) ![]() N2(g) + 2H2(g)
N2(g) + 2H2(g)
��ʼ�� 1 0 0
�仯�� X X 2X
ƽ���� 1-X X 2X
���ݷ�Ӧ 3N2H4(g) ![]() 4NH3(g) + N2(g)
4NH3(g) + N2(g)
��ʼ�� 1-X 0 2X
�仯�� 0.3 0.4 0.1
ƽ���� 1-X-0.3 0.4 0.1+X
���������Ϣ��֪��2X=0.4��X=0.2mol,���Դﵽƽ��ʱ��c(N2H4)= 1-0.2-0.3=0.5mol/L��c(N2)= 0.1+0.2=0.3 mol/L��c(H2)=2��0.2=0.4 mol/L�����¶��·�Ӧ I��ƽ�ⳣ��KC=��0.4��2��0.3/0.5=0.096����ȷ�𰸣�0.096��
�۶���N2H4(g) ![]() N2(g) + 2H2(g) ��H1 = -50.6 kJ��mol-1 ��Ӧ�������¶ȣ�ƽ�����ƣ�H2��������ļ�С������ͼ�����£�
N2(g) + 2H2(g) ��H1 = -50.6 kJ��mol-1 ��Ӧ�������¶ȣ�ƽ�����ƣ�H2��������ļ�С������ͼ�����£� ����ȷ�𰸣�
����ȷ�𰸣�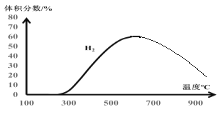 ��
��
��3����⺬��NH4+����Һ��-3�۵����ߵ�0�ۣ���Ԫ����+1�۽��͵�0�ۣ��õ����̵��ܷ�Ӧ����ʽ��![]() ����ȷ�𰸣�
����ȷ�𰸣�![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
