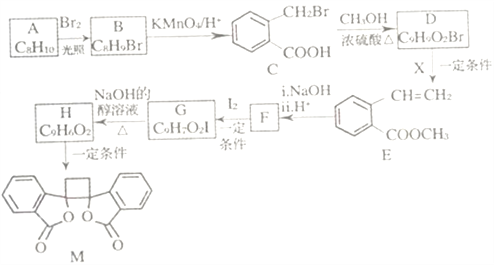
��Ŀ����
����Ŀ����. ���෨���о���ѧ���ʺͻ�ѧ��Ӧ����Ҫ�����壬������������⡣�����������ʣ� ������ ���ۻ���KNO3 ������ ��NaCl���� ��Һ̬�Ȼ��� ��ʯ��ˮ ���Ҵ��������ܵ����������_______�����ڵ���ʵ���____�����ڷǵ���ʵ���__��д����������̼��������Һ��Ӧ�����ӷ���ʽ��_____��
��.ʵ������Ҫ0.1mol/LNaOH��Һ450mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⡣
��1������ͼ��ʾ�����У�����������Һ�϶�����Ҫ����_________������ţ�����ͼ�����������⣬����������Һ����Ҫ�IJ��������� _____________________________��
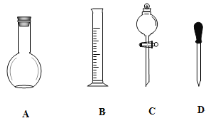
��2��������ƿ��ʹ�÷����У����в�������ȷ����____________
A��ʹ������ƿǰ�����Ƿ�©ˮ
B������ƿ��ˮϴ�������ô�����Һϴ��
C��������Һʱ����������ǹ��壬�ѳƺõĹ�����ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ��̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ�
D��������Һʱ����������Һ�壬����Ͳȡ�����ò�����������������ƿ�У�������ˮ���̶���1~2cm�����ý�ͷ�ιܼ�����ˮ���̶��ߡ�
E���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȡ�
��3�����ݼ�����������ƽ��ȡNaOH���������Ϊ_______g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��______0.1mol/L������ڡ���С�ڡ����ڡ�����
��4������18.4mol/L��Ũ����������ƣ������Ũ��������Ϊ____________mL��
���𰸡��٢ڢ� �ڢܢ� �� Ca2++2OH-+2HCO3-=CaCO3��+H2O+CO32- AC 500mL����ƿ���ձ��������� BCD 2.0g С�� 13.6
��������
��.�������ʵ����������ϵ���ʺͷǵ���ʵĸ�����������
��.(1)��������һ�����ʵ���Ũ�ȵ���Һʵ����Ҫ�IJ����ж���Ҫ���������������
(2)��������ƿ��ʹ�÷�����ע�������������ж���
(3)���ݹ�ʽm=nM=cvM�����㣻���ݶ���ʱ�۵�����Ҫ�Ͱ�Һ�����ʹ���ƽ������
(4)����ϡ��ǰ�����ʵ����������з��������㡣
��.�������ǽ������ʣ��ܵ��磬���ǼȲ��ǵ����Ҳ���Ƿǵ���ʣ����ۻ���KNO3�ܵ��磬���ڻ�������ڵ���ʣ����������ܵ��磬���ڵ��ʣ��Ȳ��ǵ����Ҳ���Ƿǵ���ʣ���NaCl���岻�ܵ��磬����ˮ������״̬���ܵ��磬���ڵ���ʣ���Һ̬�Ȼ��ⲻ�ܵ��磬����ˮ�ܵ��磬���ڵ�⣻��ʯ��ˮ�ܵ��磬���ڻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ����Ҵ����ܵ��磬���ڷǵ���ʣ��ܵ���������Т٢ڢޣ����ڵ���ʵ��Тڢܢݣ����ڷǵ���ʵ��Тߣ�����ʯ��ˮ������̼��������Һ��Ӧ��Ca2++2OH-+2HCO3-=CaCO3��+2H2O+CO32-���ʴ�Ϊ���٢ڢ����ڢܢ�������Ca2++2OH-+2HCO3-=CaCO3��+2H2O+CO32-��
��.(1)����һ�����ʵ���Ũ�ȵ���Һ������������У�һ����������ƿ��������ƽ���ձ�������������ͷ�ιܡ���Ͳ���ò�����ƿ�ͷ�Һ©������ȱ�ٵ��������ձ�����������500mL����ƿ���ʴ�Ϊ��AC���ձ�����������500mL����ƿ��
(2)A������ƿ�ڲ������ӣ�ʹ������ƿǰ�����Ƿ�©ˮ����ȷ��B������ƿ��ˮϴ�������ô�����Һϴ�ӣ�����C������ƿ������Ϊϡ�ͺ��ܽ������������D������ƿ������Ϊϡ�ͺ��ܽ������������E����������ƿʹ��ʱ��ע�����ҡ������ƿ��Ӧ�øǺ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȣ���ȷ���ʴ�Ϊ��BCD��
(3)ʵ����û��450mL������ƿ��Ӧѡ��500mL������ƿ�����ƣ��ݹ�ʽm=nM=cvM=0.1mol/L��0.5L��40g/mol=2.0g��������ʱ���ӿ̶��ߣ���ʵ����Һ�������500mL��������Ũ��ƫС���ʴ�Ϊ��2.0g��С�ڣ�
(4)��Ũ��������ΪVmL��ϡ��ǰ������������������䣬��98%��1.84g/cm3V=0.5mol/L��0.50L��98g/mol�����V=13.6mL���ʴ�Ϊ��13.6��

����Ŀ��һ���¶����������������Ϊ2.0L�ĺ����ܱ������зֱ����һ������X��������Ӧ��pX(g) ![]() Y(g)+Z(g)������������±���ʾ��
Y(g)+Z(g)������������±���ʾ��
������� | �¶�(��) | ��ʼ���ʵ���(mol) | ƽ�����ʵ���(mol) | |
X(g) | Y(g) | Z(g) | ||
�� | 387 | 0.20 | 0.080 | 0.080 |
�� | 387 | 0.40 | 0.160 | 0.160 |
�� | T | 0.20 | 0.090 | 0.090 |
�ش��������⣺
��1�����������з�Ӧ��10min�ﵽƽ�⣬��ǰ10min��Y��ƽ����Ӧ����v(Y)=___________��������������������ʼʱX�ķ�Ӧ����v(X)��___________v(X)��(������������С��������������)��
��2����֪������ӦΪ���ȷ�Ӧ����T___________387(��������������С����)���ж�������___________��
��3����Ӧ����ʽ��X�Ļ�ѧ������p��ȡֵΪ___________����������X��ƽ��ת����Ϊ___________������ʼʱ���������г���0.1molX��0.15molY��0.10molZ����Ӧ����___________ (����������������)��Ӧ����������ж�������_____________________________________________________��