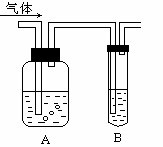
��Ŀ����
��11�֣���1����ʵ�����������Ũ���������������Ӧ��ȡ��������Ӧ�Ļ�ѧ����ʽΪ��______________________________________________________��
��2��������������Һ�м���������̷�ĩ���Բ����������˷�Ӧ�ж������̵�������_____________��
��3����SO2��CaO��D2��HCl��Na2CO3���ִ������У����ڹ��ۻ��������_________________________�����Ǽ��Լ�����_______________��
��4����֪��2 SO2 + O2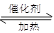 2 SO3����һ�ܱ������г���SO2��18O2����Ӧһ��ʱ���18O��������_______________________��
2 SO3����һ�ܱ������г���SO2��18O2����Ӧһ��ʱ���18O��������_______________________��
��5�����·�Ӧ��
�� Zn + CuSO4 = ZnSO4 +Cu �� NaOH + HCl =" NaCl" + H2O
�� 4Al + 3O2 = 2Al2O3 �� Na2CO3 + H2SO4 = Na2SO4 + CO2��+ H2O
���п�����Ƴ�ԭ��ص���_______________��д��ţ���
��2��������������Һ�м���������̷�ĩ���Բ����������˷�Ӧ�ж������̵�������_____________��
��3����SO2��CaO��D2��HCl��Na2CO3���ִ������У����ڹ��ۻ��������_________________________�����Ǽ��Լ�����_______________��
��4����֪��2 SO2 + O2
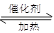 2 SO3����һ�ܱ������г���SO2��18O2����Ӧһ��ʱ���18O��������_______________________��
2 SO3����һ�ܱ������г���SO2��18O2����Ӧһ��ʱ���18O��������_______________________����5�����·�Ӧ��
�� Zn + CuSO4 = ZnSO4 +Cu �� NaOH + HCl =" NaCl" + H2O
�� 4Al + 3O2 = 2Al2O3 �� Na2CO3 + H2SO4 = Na2SO4 + CO2��+ H2O
���п�����Ƴ�ԭ��ص���_______________��д��ţ���
��1��MnO2 + 4HCl��Ũ��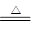 MnCl2 + Cl2��+2H2O��2�֣�
MnCl2 + Cl2��+2H2O��2�֣�
��2����������1�֣� ��3��SO2��HCl��2�֣���D2��1�֣�
��4��O2��SO2��SO3��3�֣� ��5���٢ۣ�2�֣�
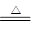 MnCl2 + Cl2��+2H2O��2�֣�
MnCl2 + Cl2��+2H2O��2�֣���2����������1�֣� ��3��SO2��HCl��2�֣���D2��1�֣�
��4��O2��SO2��SO3��3�֣� ��5���٢ۣ�2�֣�
��1�����������ڼ���ʱ��������Ũ������������������ʽΪMnO2 + 4HCl��Ũ��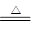 MnCl2 + Cl2��+2H2O��
MnCl2 + Cl2��+2H2O��
��2�����������ֽܷ�����������ˮ���ڴ����������£����Լӿ�ֽ����ʣ���˶�����������������á�
��3��ȫ���ɹ��ۼ��γɵĻ������ǹ��ۻ��������SO2��HCl�ǹ��ۻ���������ƺ�̼���������ӻ������ͬһ�ַǽ����γɵĹ��ۼ��ǷǼ��Լ�������D2���ɷǼ��Լ��γɵĵ��ʡ�
��4����Ϊ�ǿ��淴Ӧ���������������������ͬʱ�����������ַֽ����������Ͷ�������O2��SO2��SO3�о�����18O��
��5��ԭ������е��Ӷ����ƶ�������ֻ��������ԭ��Ӧ������Ƴ�ԭ��أ����٢ۿ��ԡ�
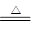 MnCl2 + Cl2��+2H2O��
MnCl2 + Cl2��+2H2O����2�����������ֽܷ�����������ˮ���ڴ����������£����Լӿ�ֽ����ʣ���˶�����������������á�
��3��ȫ���ɹ��ۼ��γɵĻ������ǹ��ۻ��������SO2��HCl�ǹ��ۻ���������ƺ�̼���������ӻ������ͬһ�ַǽ����γɵĹ��ۼ��ǷǼ��Լ�������D2���ɷǼ��Լ��γɵĵ��ʡ�
��4����Ϊ�ǿ��淴Ӧ���������������������ͬʱ�����������ַֽ����������Ͷ�������O2��SO2��SO3�о�����18O��
��5��ԭ������е��Ӷ����ƶ�������ֻ��������ԭ��Ӧ������Ƴ�ԭ��أ����٢ۿ��ԡ�

��ϰ��ϵ�д�
�����Ŀ