��Ŀ����
��12�֣���ҵ��һ��ɲ������·�Ӧ���ϳɼ״���
CO(g)+2H2(g)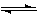 CH3OH(g) ��H����a kJ��mol��1
CH3OH(g) ��H����a kJ��mol��1
��1����ͼ�Ǹ÷�Ӧ�ڲ�ͬ�¶���CO��ת������ʱ��仯�����ߡ�
��a __0������� ������ ����������
������˵����ȷ����_ _������ţ���
a��1mol CO(g)��2mol H2(g)�����������1mol CH3OH(g)���������
b����1mol CO(g)��2mol H2(g)����һ�ܱ������г�ַ�Ӧ��ų�a KJ������
c�������¶ȣ�ƽ�����淴Ӧ�ƶ��������Ȼ�ѧ����ʽ�е�aֵ����С
d���罫һ����CO(g) ��H2(g)����ij�ܱ������г�ַ�Ӧ�����aKJ����˹�������1molCO(g)����ԭ
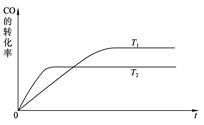
��2����һ�������£���ѧ�����ô��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״������������ͼ��ʾ��
���úϳ�·�߶��ڻ��������ļ�ֵ����_ _��
��15%��20%���Ҵ�����HOCH2CH2NH2��ˮ��Һ���������ԣ������ϳ���·������CO2
���ռ��������ӷ���ʽ��ʾ�Ҵ���ˮ��Һ�������Ե�ԭ��
��
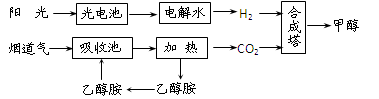
��3���״�ȼ�ϵ�صĹ���ԭ��������ͼ��ʾ���õ�ع���ʱ��c��ͨ������ʷ����ĵ缫
��ӦʽΪ��_ _��
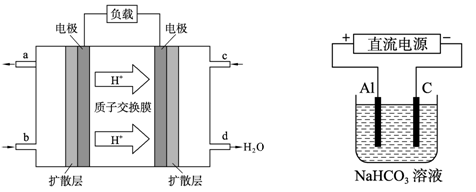
��4���������������Դ��������ͼ��ʾװ�ã���ʵ������ģ������Ʒ���桰�ۻ��������Ĺ����У�������Һ����ǣ�ԭ���ǣ�����صĵ缫��Ӧʽ�����ӷ���ʽ��ʾ����
_ ��
CO(g)+2H2(g)
 CH3OH(g) ��H����a kJ��mol��1
CH3OH(g) ��H����a kJ��mol��1
��1����ͼ�Ǹ÷�Ӧ�ڲ�ͬ�¶���CO��ת������ʱ��仯�����ߡ�
��a __0������� ������ ����������
������˵����ȷ����_ _������ţ���
a��1mol CO(g)��2mol H2(g)�����������1mol CH3OH(g)���������
b����1mol CO(g)��2mol H2(g)����һ�ܱ������г�ַ�Ӧ��ų�a KJ������
c�������¶ȣ�ƽ�����淴Ӧ�ƶ��������Ȼ�ѧ����ʽ�е�aֵ����С
d���罫һ����CO(g) ��H2(g)����ij�ܱ������г�ַ�Ӧ�����aKJ����˹�������1molCO(g)����ԭ
��2����һ�������£���ѧ�����ô��̵����з����CO2��̫���ܵ�ص��ˮ������H2�ϳɼ״������������ͼ��ʾ��

���úϳ�·�߶��ڻ��������ļ�ֵ����_ _��
��15%��20%���Ҵ�����HOCH2CH2NH2��ˮ��Һ���������ԣ������ϳ���·������CO2
���ռ��������ӷ���ʽ��ʾ�Ҵ���ˮ��Һ�������Ե�ԭ��
��
��3���״�ȼ�ϵ�صĹ���ԭ��������ͼ��ʾ���õ�ع���ʱ��c��ͨ������ʷ����ĵ缫
��ӦʽΪ��_ _��

��4���������������Դ��������ͼ��ʾװ�ã���ʵ������ģ������Ʒ���桰�ۻ��������Ĺ����У�������Һ����ǣ�ԭ���ǣ�����صĵ缫��Ӧʽ�����ӷ���ʽ��ʾ����
_ ��
��1���� >��2�֣� ��d��2�֣�
��2���������ڷ�ֹ����ЧӦ��2�֣�
��HOCH2CH2NH2 �� H2O HOCH2CH2NH3���� OH����2�֣�
HOCH2CH2NH3���� OH����2�֣�
��3�� O2+4e��+4H+ =2H2O��2�֣���
��4�� Al��3e��= Al3+��1�֣� Al3++3HCO3�� = Al(OH)3��+3CO2����2�֣�
��Al��3e��+3HCO3�� = Al(OH)3��+3CO2����2�֣�
��2���������ڷ�ֹ����ЧӦ��2�֣�
��HOCH2CH2NH2 �� H2O
 HOCH2CH2NH3���� OH����2�֣�
HOCH2CH2NH3���� OH����2�֣���3�� O2+4e��+4H+ =2H2O��2�֣���
��4�� Al��3e��= Al3+��1�֣� Al3++3HCO3�� = Al(OH)3��+3CO2����2�֣�
��Al��3e��+3HCO3�� = Al(OH)3��+3CO2����2�֣�
�������������ƽ���Ӱ���Լ��绯ѧ��Ӧ�õȡ�
��1������ͼ���֪���¶�ΪT2�������ȴﵽƽ��״̬������T2����T1�������¶ȵ����ߣ���Ӧ���ת�����ǽ��͵ģ�˵�������¶�ƽ�����淴Ӧ�����ƶ�����������Ӧ�Ƿ��ȷ�Ӧ��a����0����Ӧ���ȣ�˵����Ӧ����������������������������a����ȷ����Ӧ�ǿ��淴Ӧ��1mol CO(g)��2mol H2(g)����������1mol�״������Էų�������ҪС��akJ��b����ȷ����Ӧ��������ǰ��Ļ�ѧ�������йأ���ƽ����ƶ������أ�c����ȷ������ѡ��d����ȷ�ġ�
��2������ת����֪���ɵ�CO2�����ϳɼ״������������ڿ�֪����ЧӦ���������᰷�Ľṹ��ʽ��֪�������еİ������Խ��ˮ������������ӣ��Ӷ��ƻ�ˮ�ĵ���ƽ�⣬ʹ��Һ��OH��Ũ�ȴ���������Ũ�ȣ���Һ�Լ��ԡ�
��3��ԭ����и�����ʧȥ���ӵģ��״��ڷ�Ӧ���ǻ�ԭ��ʧȥ���ӱ���������˼״��ڸ���ͨ�롣����ȼ�ϵ�صĽṹ���ж����������Ҳ��ƶ��������Ҳ������������缫�Ǹ���������������Ҳ�ͨ�룬����c���ĵ缫��ӦʽΪO2+4e��+4H+ =2H2O��
��4�����ݵ��صĽṹ��֪������������ʧȥ�������������ӽ�����Һ�С����ڵ������̼��������Һ���������ɵ������Ӻ�̼������ˮ����ٽ����Ӷ�������������������
��1������ͼ���֪���¶�ΪT2�������ȴﵽƽ��״̬������T2����T1�������¶ȵ����ߣ���Ӧ���ת�����ǽ��͵ģ�˵�������¶�ƽ�����淴Ӧ�����ƶ�����������Ӧ�Ƿ��ȷ�Ӧ��a����0����Ӧ���ȣ�˵����Ӧ����������������������������a����ȷ����Ӧ�ǿ��淴Ӧ��1mol CO(g)��2mol H2(g)����������1mol�״������Էų�������ҪС��akJ��b����ȷ����Ӧ��������ǰ��Ļ�ѧ�������йأ���ƽ����ƶ������أ�c����ȷ������ѡ��d����ȷ�ġ�
��2������ת����֪���ɵ�CO2�����ϳɼ״������������ڿ�֪����ЧӦ���������᰷�Ľṹ��ʽ��֪�������еİ������Խ��ˮ������������ӣ��Ӷ��ƻ�ˮ�ĵ���ƽ�⣬ʹ��Һ��OH��Ũ�ȴ���������Ũ�ȣ���Һ�Լ��ԡ�
��3��ԭ����и�����ʧȥ���ӵģ��״��ڷ�Ӧ���ǻ�ԭ��ʧȥ���ӱ���������˼״��ڸ���ͨ�롣����ȼ�ϵ�صĽṹ���ж����������Ҳ��ƶ��������Ҳ������������缫�Ǹ���������������Ҳ�ͨ�룬����c���ĵ缫��ӦʽΪO2+4e��+4H+ =2H2O��
��4�����ݵ��صĽṹ��֪������������ʧȥ�������������ӽ�����Һ�С����ڵ������̼��������Һ���������ɵ������Ӻ�̼������ˮ����ٽ����Ӷ�������������������

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ