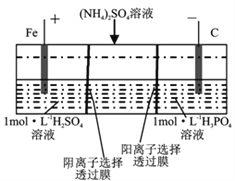
��Ŀ����
����Ŀ��������������־������־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ��������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
��1����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ______��3d�ܼ��ϵ�δ�ɶԵ�����Ϊ______��
��2�����������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����____��
����[Ni(NH3)6]SO4��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ____���ṩ�µ��ӶԵijɼ�ԭ����______��
�۰��ķе�__________������������������������좣�PH3����ԭ����_________������______���ӣ����������������Ǽ�������������ԭ�ӵĹ���ӻ�����Ϊ_______��
��3������ͭ����������_______���γɵľ��壻Ԫ��ͭ�����ĵڶ������ֱܷ�Ϊ��ICu=1958 kJ��mol�C1��INi=1 753 kJ��mol�C1��ICu> INi��ԭ����_______________��
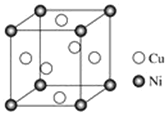
��4��ij����ͭ�Ͻ�����������ṹ��ͼ��ʾ��
�پ�����ͭԭ������ԭ�ӵ�������Ϊ________��
�����Ͻ���ܶ�Ϊd g��cm�C3����������a=______nm�����ú���d��NA��ʽ�ӱ�ʾ�����Բ�����
���𰸡�1s22s2sp63s23p63d84s2 2 �������� ��λ�� N ���� �������Ӽ������� ���� sp3 ���� Cu+3d���ȫ�������ȶ���ʧȥ�������յ��������࣬�ڶ������ܸ��� 3 ��1 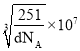
��������
��1��NiԪ��ԭ�Ӻ��������Ϊ28����������Ų�ʽΪ��1s22s2sp63s23p63d84s2��3d�ܼ��ϵ�δ�ɶԵ�����Ϊ2��
��2����[Ni(NH3)6]SO4��������������������ӣ�Sԭ����![]() =0�Թµ��Ӷԣ��γ�4���������ӻ������ĿΪ4��Sԭ�Ӳ�ȡsp3�ӻ������幹�����������塣
=0�Թµ��Ӷԣ��γ�4���������ӻ������ĿΪ4��Sԭ�Ӳ�ȡsp3�ӻ������幹�����������塣
����[Ni(NH3)6]SO4��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ��λ�����ṩ�µ��ӶԵijɼ�ԭ����N��
��PH3����֮��Ϊ���»�������������֮����˷��»��������γ���������Ӽ���������ǿ�����������ʵķе㣬�ʰ����ķе����PH3���ӣ�
NH3����Ϊ�����νṹ�����������������IJ��غϣ����ڼ��Է��ӣ�Nԭ����1�Թµ��Ӷԣ��γ�3���������ӻ������ĿΪ4����ԭ�Ӳ�ȡsp3�ӻ���
��3������ͭ���������ڽ������壬�����ɽ������γɵľ��壻Cu������Χ�����Ų�Ϊ3d10��Ni������Χ�����Ų�Ϊ3d84s1��Cu+��3d����ȫ�������ȶ���ʧȥ�ڶ������Ӹ��ѣ�Ԫ��ͭ�ĵڶ������ܸ�������
��4���پ�����Ni���ڶ��㣬Cu�������ģ�����Niԭ����ĿΪ8��![]() =1��Cuԭ����Ŀ=6��
=1��Cuԭ����Ŀ=6��![]() =3����Cu��Niԭ����Ŀ֮��Ϊ3��1��
=3����Cu��Niԭ����Ŀ֮��Ϊ3��1��
�ڸ�����=![]() ������=
������=![]() g/cm3 =
g/cm3 =![]() =d g��cm�C3����������a=
=d g��cm�C3����������a=![]() cm=
cm=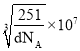 nm��
nm��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��H2O2�㷺Ӧ����ҽ����������ѧ�ϳɵ�����
��1��H2O2�ĵ���ʽ��___________��
��2��Ȥζʵ�顰�������ࡱ��ʵ��ԭ����H2O2��Һ��KI�������·ֽ⣬��Ӧ�Ļ����ɱ�ʾΪ��i��H2O2(l)+I��(aq)==== H2O(l)+IO��(aq) ��H1 = +a kJ/moL��a �� 0��
ii��H2O2(l)+________________________________��
�� 2H2O2(l)=2H2O(l)+O2(g) ��H=��196kJ/mol����ȫ��Ӧii_______________�����Ȼ�ѧ����ʽ��ʾ����
�� ijС�����о�Ӱ��H2O2�ֽ����ʵ�����ʱ�õ�ͼ1�����ݹ�ϵ���ɴ˵ó��Ľ�����________��
�� ��֪��i�ķ�Ӧ����С��ii�ķ�Ӧ���ʣ���ͼ2����H2O2��Һ�м���KI����Ӧ���̡�������ʾ��ͼ��_________

��3��Ϊ������ͬ�Լ��Ƿ��H2O2�ֽ��д���������С������֧ʢ��10mL5% H2O2���Թ��еμӲ�ͬ��Һ��ʵ���¼���£�
�Թ� | �� | �� | �� | �� |
�μ��Լ� | 2��1mol/L NaCl | 2��1mol/L CuSO4 | 2��1mol/L CuSO4 ��2��1mol/L NaCl | 2��1mol/L CuSO4 ��4��1mol/L NaCl |
����������� | �����ݲ��� | ���������ݣ����������ʴӢ����μӿ� | ||
ʵ����Ŀ����______________����ʵ�飨3���ɵó��Ľ�����________________��