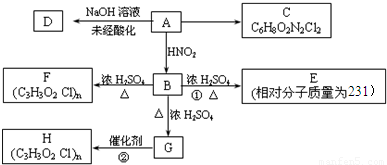
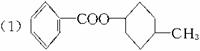��Ŀ����
��֪����һ��̼ԭ���������ĸ�ԭ�ӻ�ԭ������ȫ��ͬʱ����̼ԭ�ӽ�����̼��
��֪��������������һ����������HNO2��Ӧ�������ɴ���
R�DNH2 + NNO2![]() ROH + N2��+ H2O
ROH + N2��+ H2O
������ͼ��ʾ��ת����ϵ�����У�B��������һ������̼ԭ�ӣ�1 mol B�ֱ���������Na��������NaHCO3��Ӧ�����ų���״���µ�����22.4 L��C��������һ����Ԫ������Aÿ����1mol C��ͬʱ����2 molH2O��E��һ�־���ˮ����ζ�����ʡ�
�������ͼ�ش����⣺
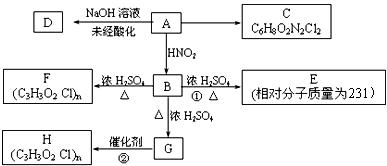
��1��B�Ľṹ��ʽΪ___________________________________��
��2��A�к��������ŵ�����Ϊ___________��C�к��������ŵ�����Ϊ_____________��
��3��д��C��D��F�Ľṹ��ʽ��
C________________ D_________________ F _________________
��4�����ж����з�Ӧ�����ͣ���Ӧ��________________ ��Ӧ��_________________��
��5��д�����з�Ӧ����ʽ��
B��G��______________________________________________________________
G��H��______________________________________________________________
��1�� ![]()
��2�� �Ȼ��� ����
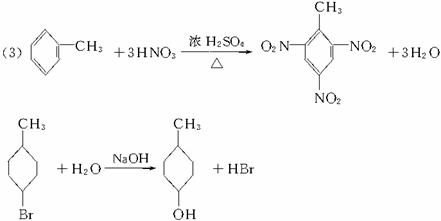
��3�� 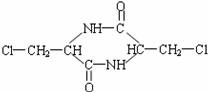
![]()
![]()
��4����������Ӧ �ڼӾ۷�Ӧ
��5��
��![]()
��![]()