��Ŀ����
����Ŀ��ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ���̪��ָʾ��������д���пհס�
��1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��______________��ֱ�������һ���������Һ��________ɫ��Ϊ________ɫ����________Ϊֹ��
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���__________��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
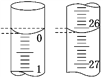
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ_______mL���յ����Ϊ_______mL������������Һ�����Ϊ_______mL��
��4��ijѧ������3��ʵ��ֱ��¼�й��������±���
�ζ� ���� | ����NaOH��Һ�����/mL | 0.100 0 mol��L��1 ��������/mL | ||
�ζ�ǰ���� | �ζ������ | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
�����ϱ�������ʽ�����NaOH��Һ�����ʵ���Ũ��__________________________��
���𰸡� ��ƿ����Һ��ɫ�仯 �� �� ������ڲ��ָ�ԭ������ɫ D 0.00 26.10 26.10 0.1044mol/L
��������(1)����к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯�����ж��յ㣬�������������ǡ�÷�Ӧ��Һ�����ԣ�ѡ����Ա�ɫ��Χ�ڵ�ָʾ����̪���ζ��յ�ʱ��Һ��ɫ�ɺ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ��ָ�ԭ������ɫ���ʴ�Ϊ����ƿ����Һ����ɫ�仯���죻�ޣ�������ڲ��ָ�ԭ������ɫ��
(2)A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��Ũ��ƫС�����V(��)ƫ����c(����)= 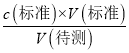 �������ⶨc(����)ƫ��A����B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V(��)��Ӱ�죬����c(����)=
�������ⶨc(����)ƫ��A����B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V(��)��Ӱ�죬����c(����)=  �������ⶨc(����)��Ӱ�죬��B����C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V(��)ƫ����c(����)=
�������ⶨc(����)��Ӱ�죬��B����C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V(��)ƫ����c(����)= 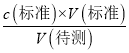 �������ⶨc(����)ƫ��C����D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V(��)ƫС������c(����)=
�������ⶨc(����)ƫ��C����D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V(��)ƫС������c(����)= 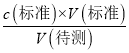 �������ⶨc(����)ƫС����D��ȷ����ѡD��
�������ⶨc(����)ƫС����D��ȷ����ѡD��
(3)��ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��������Һ�����Ϊ26.10mL���ʴ�Ϊ��0.00��26.10��26.10��
(4)�������ݵ���Ч�ԣ���ȥ��2�����ݣ���1��3��ƽ������V(����)= ![]() =26.10mL�����ݷ�Ӧ����ʽHCl+NaOH�TNaCl+H2O����c(NaOH)=
=26.10mL�����ݷ�Ӧ����ʽHCl+NaOH�TNaCl+H2O����c(NaOH)= ![]() =0.1044mol/L����NaOH��Ũ��Ϊ0.1044mol/L��
=0.1044mol/L����NaOH��Ũ��Ϊ0.1044mol/L��

����Ŀ��������ʵ�鼰�������Ƴ���Ӧ���۵���
ʵ�� | ���� | ���� | |
A | ��2mL0.1mol��L-1��FeCl3��Һ�м��������ۣ�����1��KSCN��Һ | ��ɫ������ʧ����KSCN��Һ��ɫ���� | ��ԭ�ԣ�Fe>Fe2+ |
B | ����������ȼ�ճ��е�ȼ��Ѹ�����뼯��CO2�ļ���ƿ | ����ƿ�в����������̣�ƿ���к�ɫ�������� | CO2���������� |
C | ����ʢ����NH4HCO3������Թܣ������Թܿڷ���ʪ��ĺ�ɫʯ����ֽ | ʯ����ֽ���� | NH4HCO3�Լ��� |
D | ��2֧ʢ��2mL��ͬŨ��������Һ���Թ��зֱ����2����ͬŨ�ȵ�NaCl��NaI��Һ | һֻ�Թ��в�����ɫ��������һ֧������������ | Ksp(AgI)<Ksp(AgCl) |
A. A B. B C. C D. D