��Ŀ����
(1)�й��Ŵ��Ĵ���֮һ�����ڻ�ҩ�����ı�ը��ӦΪ
2KNO3
��3C��S��A��N2��3CO2��(����ƽ)�ٳ�S�⣬����Ԫ�صĵ縺�ԴӴ�С����Ϊ________��
�����������У�A�Ļ�ѧ������Ϊ________�������Թ��ۼ��ķ��ӵ�����ԭ�ӹ���ӻ�����Ϊ________����________���ӣ�
����֪CN����N2�ṹ���ƣ�����HCN�����ЦҼ���м���Ŀ֮��Ϊ________��
(2)ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��ԭ������T��Q��2��T�Ļ�̬ԭ����Χ����(�۵���)�Ų�Ϊ________��Q2+��δ�ɶԵ�������________��
(3)��CrCl3��ˮ��Һ�У�һ�������´������Ϊ[CrCln(H2O)6��n]x+(n��x��Ϊ������)�������ӣ�����ͨ�������ӽ�����֬(R��H)���ɷ������ӽ�����Ӧ��
[CrCln(H2O)6��n]x+��xR��H![]() Rx[CrCln(H2O)6��n]x+��xH+
Rx[CrCln(H2O)6��n]x+��xH+
����������H+���к͵ζ����������x��n��ȷ�������ӵ���ɣ�����0.0015 mol��[CrCln(H2O)6��n]x+����Һ����R��H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L��1��NaOH��Һ25.00 ml���������ӵĻ�ѧʽΪ________��
(4)�������������Ļ����15 g�м���ϡ����150 mL����״���·ų�����1.68 L��ͬʱ��������������ʣ�࣮����Һ�е���KSCNδ����ɫ�仯��Ϊ���к��������ᣬ��ʹFe2+��ȫת��Ϊ������������������3 mol/L������������Һ200 mL����ԭ��������ʵ���Ũ��Ϊ________mol/L��
������
����
(1)��O��N��C��K���������Ӽ���SP�ӻ����ڡ��Ǽ��Է��ӣ�
������1��1
����(2)3d84s2��4
����(3)��ѧʽΪ[CrCl(H2O)5]2+
����
(4)2 mol/L��
 �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�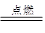 A+N2��+3CO2��������ƽ��
A+N2��+3CO2��������ƽ�� A+N2��+3CO2��������ƽ��
A+N2��+3CO2��������ƽ��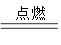 A+N2��+3CO2��������ƽ��
A+N2��+3CO2��������ƽ��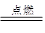 A+N2��+3CO2��������ƽ��
A+N2��+3CO2��������ƽ�� A+ N2�� +3CO2��
A+ N2�� +3CO2��