��Ŀ����
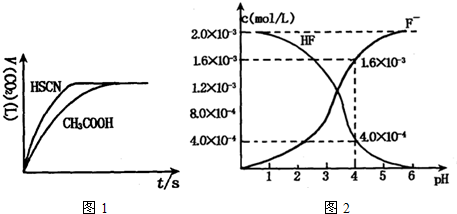
��8�֣���1����֪25��ʱ������ʵ���ƽ�ⳣ����Ka��CH3COOH����1.8��10-5��Ka��HSCN����0.13��25��ʱ����20mL 0��10 mol��L��1 CH3COOH��Һ��20mL 0��10 mol��L��1HSCN��Һ�ֱ���20mL 0��10 mol��L��1NaHCO3��Һ��ϣ�ʵ���ò��������������V����ʱ�䣨t���仯��ʾ��ͼΪ��
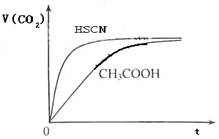
��Ӧ��ʼ�Σ�������Һ����CO2��������ʴ������Բ����ԭ���� ��
��Ӧ��������������Һ�У�c��CH3COO���� c��SCN�������������������������
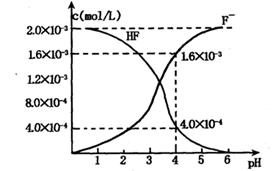
��2��25��ʱ����pH=1��H2SO4��Һa mL��pH=12��NaOH��Һb mL��Ϻ�������Һ��pH=3����a��b= ����Ӧ����Һ�и�����Ũ���ɴ�С��˳���� ��
��1��HSCN�����Ա�CH3COOHǿ������Һ��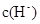 �ϴ�����Һ��NaHCO3��Һ�ķ�Ӧ���ʿ�
�� ��2��1 : 9��c��Na+��>c��SO
�ϴ�����Һ��NaHCO3��Һ�ķ�Ӧ���ʿ�
�� ��2��1 : 9��c��Na+��>c��SO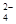 ��>c��H+��>c��OH����
��>c��H+��>c��OH����
����������1�����ݵ��볣����֪��HSCN�����Ա�CH3COOHǿ������Һ��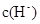 �ϴ�����Һ��NaHCO3��Һ�ķ�Ӧ���ʿ졣��Խ������Ӧ������Խ����ˮ�⡣����������Һ�ļ���ǿ��NaSCN��Һ�ļ��ԡ�����c��CH3COO����С��c��SCN������
�ϴ�����Һ��NaHCO3��Һ�ķ�Ӧ���ʿ졣��Խ������Ӧ������Խ����ˮ�⡣����������Һ�ļ���ǿ��NaSCN��Һ�ļ��ԡ�����c��CH3COO����С��c��SCN������
��2�������������������������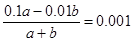 �����a��b=1 : 9�����ݵ���غ�c��Na+���� c��H+����2c��SO
�����a��b=1 : 9�����ݵ���غ�c��Na+���� c��H+����2c��SO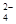 ����c��OH������֪����Һ�и�����Ũ���ɴ�С��˳����c��Na+��>c��SO
����c��OH������֪����Һ�и�����Ũ���ɴ�С��˳����c��Na+��>c��SO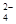 ��>c��H+��>c��OH������
��>c��H+��>c��OH������

 ��У����ϵ�д�
��У����ϵ�д�