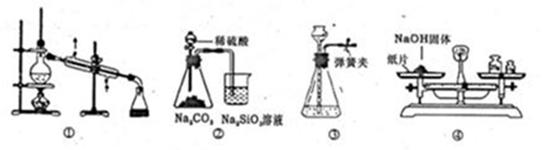
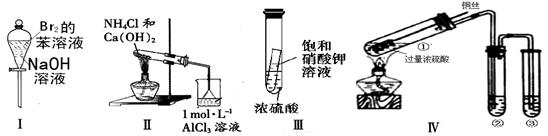
��Ŀ����
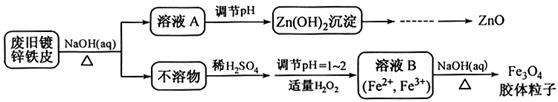
������п�к�����CuO��Fe3O4��SiO2�����ʡ���ҵ���Դ�����п��������п���壨ZnSO4��7H2O���Ĺ�����������ͼ��ʾ��
 ��
��
��֪�������£���Һ�е�Fe3+��Zn2+��Fe2+������������ʽ��ȫ������pH�ֱ�Ϊ��3.7��6.5��9.7��
��ش��������⣺
��1�������۵������� ��
��2����������п�۵�����Ϊ ��
��3������30%H2O2��Ӧ�����ӷ���ʽ�� ��
��4����������Ca(OH)2������ҺpH���ٽ�Fe3+ˮ�⣬Fe3+ˮ�ⷴӦ��ƽ�ⳣ������ʽK���� ����Ca(OH)2���ܹ�����ԭ������ ����
 ��
����֪�������£���Һ�е�Fe3+��Zn2+��Fe2+������������ʽ��ȫ������pH�ֱ�Ϊ��3.7��6.5��9.7��
��ش��������⣺
��1�������۵������� ��
��2����������п�۵�����Ϊ ��
��3������30%H2O2��Ӧ�����ӷ���ʽ�� ��
��4����������Ca(OH)2������ҺpH���ٽ�Fe3+ˮ�⣬Fe3+ˮ�ⷴӦ��ƽ�ⳣ������ʽK���� ����Ca(OH)2���ܹ�����ԭ������ ����
����14�֣�
��1������Ũ������ȴ�ᾧ�����˸���(ÿ��1�֣���3��)��
��2��ʹ��Һ�е�Fe3+ת��ΪFe2+(2��)����ȥCu2+(2��)
��3��2Fe2+ + H2O2 + 2H+ = 2Fe3+ + 2H2O (3��)
��4��c3(H+)/c(Fe3+) ��2�֣��� ��ֹ����Zn(OH)2��2�֣�
��1������Ũ������ȴ�ᾧ�����˸���(ÿ��1�֣���3��)��
��2��ʹ��Һ�е�Fe3+ת��ΪFe2+(2��)����ȥCu2+(2��)
��3��2Fe2+ + H2O2 + 2H+ = 2Fe3+ + 2H2O (3��)
��4��c3(H+)/c(Fe3+) ��2�֣��� ��ֹ����Zn(OH)2��2�֣�
���������ZnO��CuO��Fe3O4��SiO2���ڷ�Ӧ1��SiO2����H2SO4�����ˣ������٣���õ���Fe3+��Zn2+��Fe2+��H+��SO42-��Һ����������п��2Fe3++Zn= 2Fe2+ +Zn2+��Cu2++ Zn= Cu +Zn2+�����ˣ������ڣ���Zn2+��Fe2+��SO42-��Һ������30%H2O2ʹFe2+ת��ΪFe3+����������Ca(OH)2������ҺpH��ʹFe3+�������ɵ�Zn SO4��Һ��
��ZnSO4��Һ�õ�����п���壨ZnSO4��7H2O��Ӧ��������Ũ������ȴ�ᾧ�����˸��
п�ۿ��Է���2Fe3++Zn= 2Fe2+ +Zn2+��Cu2++ Zn= Cu +Zn2+�ķ�Ӧ��
���������Ϣ2Fe2+ + H2O2 + 2H+ = 2Fe3+ + 2H2O��
��Fe3+ +3H2O
 Fe (OH)3+3H+ K= c3(H+)/c(Fe3+)����Ca(OH)2����ʹ��Һ�ʼ���ʱZn2+����ȫ����Zn(OH)2������
Fe (OH)3+3H+ K= c3(H+)/c(Fe3+)����Ca(OH)2����ʹ��Һ�ʼ���ʱZn2+����ȫ����Zn(OH)2������
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

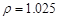 g/cm3 )����ȡ��36.5% (
g/cm3 )����ȡ��36.5% (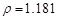 g/cm3 )������ mL
g/cm3 )������ mL