��Ŀ����
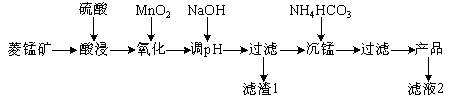
����Ŀ����ij���̿�(��MnCO3��SiO2��FeCO3������Al2O3��)Ϊԭ��ͨ�����·����ɻ��̼���̴ֲ�Ʒ��
(��֪��Ksp(MnCO3)��2.2��10��11��Ksp[Mn(OH)2]��1.9��10��13��Ksp[Al(OH)3]��1.3��10��33��Ksp[Fe(OH)3]��4.0��10��38)
��1������1�У�����Ԫ�ص�������Ҫ�� (�ѧʽ����ͬ)����NaOH������Һ��pHԼΪ5�����pH�����ܵ�������1�� �������١�
��2����Һ2�У���1�������ӳ���H����� (�����ӷ���)��
��3��ȡ��������ǰ��Һa mL����ƿ�У���������AgNO3��Һ(������)������1.5%(NH4)2S2O8��Һ�����ȣ�Mn2��������ΪMnO����Ӧһ��ʱ��������5 min[��ȥ������(NH4)2S2O8]����ȴ�����¡�ѡ�����˵�ָʾ������b mol��L��1��(NH4)2Fe(SO4)2����Һ�ζ����յ㣬����(NH4)2Fe(SO4)2����ҺV mL��
��Mn2����(NH4)2S2O8��Ӧ�Ļ�ԭ����Ϊ (�ѧʽ)��
�ڡ�������ǰ��Һ��c(Mn2��)�� mol��L��1��
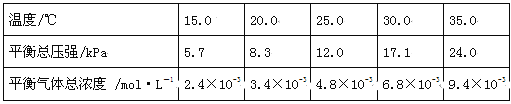
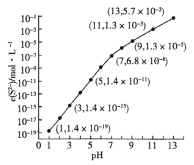
��4�������������䣬����������������Ԫ�ػ�������NH4HCO3��ʼŨ��(c0)����Ӧʱ��Ĺ�ϵ����ͼ��ʾ��

��NH4HCO3��ʼŨ��Խ����Ԫ�ػ�����Խ (����������������)������ԭ�� ��
������Һ��c(Mn2��)��1.0 mol��L��1����������1.8 mol��L��1 NH4HCO3��Һ���з�Ӧ������20��40 min��v(Mn2��)�� ��
���𰸡�
��1��Fe(OH)3��Al(OH)3��SiO2��
��2��Na����NH��
��3���� SO[����H2SO4����Na2SO4����(NH4)2SO4��]�� �� ![]()
��4���������� c(NH4HCO3)Խ��c(CO)Խ��ʹƽ��MnCO3(s)![]() CO(aq)��Mn2��(aq)�����ƣ�������MnCO3Խ����
CO(aq)��Mn2��(aq)�����ƣ�������MnCO3Խ����
��5��7.5��10��3mol��L��1��min��1
��������
������������̿���ϡ�����ܽ⣬MnCO3��FeCO3��Al2O3����ϡ���ᷴӦ���������Σ�SiO2����Ӧ�����������£�����MnO2��Fe2+����ΪFe3+��MnO2��ԭ����Mn2+��������Һ��pH��ʹFe3+��Al3+��ȫת��ΪFe(OH)3��Al(OH)3���������˷��룬����1ΪSiO2��Al(OH)3��Fe(OH)3����Һ�д��� MnSO4�������Ƶȣ�����̼����淋õ�MnCO3�����˷��룬��Һ2�к�������李������Ƶ���
��1������1�У�����Ԫ�ص�������Ҫ��Fe(OH)3����NaOH������Һ��pHԼΪ5�����pH�����ܵ�������1��SiO2��Al(OH)3�ܽ⣬��������1��SiO2��Al(OH)3��С���ʴ�Ϊ��Fe(OH)3��SiO2��Al(OH)3��
��2����Һ2�к�������李������Ƶȣ�+1�������ӳ���H+��У�Na+��NH4+���ʴ�Ϊ��Na+��NH4+��
��3����Mn2+��������(NH4)2S2O8��SԪ�ػ�ԭΪSO42-���ʴ�Ϊ��SO42-��
����MnԪ���غ㡢����ת���غ㣬�ɵù�ϵʽ��Mn2+��MnO4-��5Fe2+����n(Mn2+)=![]() n(Fe2+)������c(Mn2+)=
n(Fe2+)������c(Mn2+)=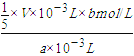 =
=![]() mol/L���ʴ�Ϊ��
mol/L���ʴ�Ϊ��![]() ��
��
��4������ͼ��֪��NH4HCO3��ʼŨ��Խ����Ԫ�ػ�����Խ�ߣ�NH4HCO3��ʼŨ��Խ����Һ��c(CO32-)��Խ�����ܶȻ�Ksp(MnCO3)=c(Mn2+)��c(CO32-)��֪��Һc(Mn2+)ԽС��������MnCO3Խ�࣬�ʴ�Ϊ���ߣ�NH4HCO3��ʼŨ��Խ����Һ��c(CO32-)��Խ�����ܶȻ�Ksp(MnCO3)=c(Mn2+)��c(CO32-)��֪��Һc(Mn2+)ԽС��������MnCO3Խ�ࣻ
������Һ��c(Mn2+)=1.0 molL-1����������![]() ��(50%-20%)=0.15mol/L����20��40 min��v(Mn2+)=
��(50%-20%)=0.15mol/L����20��40 min��v(Mn2+)=![]() =0.0075mol/(L��min)���ʴ�Ϊ��0.0075mol/(L��min)��
=0.0075mol/(L��min)���ʴ�Ϊ��0.0075mol/(L��min)��