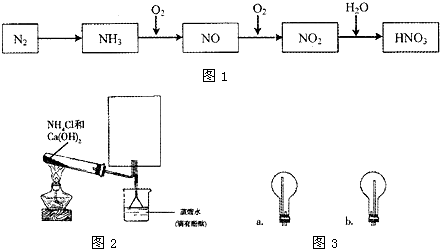
��Ŀ����
Na2O2��NO2�ܷ�����Ӧ��ij��ȤС��Դ˽���̽������������ּ��裮
����һ��Na2O2+2NO2=2NaNO2+O2��
�������Na2O2+2NO2=2NaNO3
[��������]
2NaNO2+2HCl=2NaCl+NO��+NO2��+H2O
2NO2+2NaOH=NaNO2+NaNO3+H2O
[ʵ��̽��]
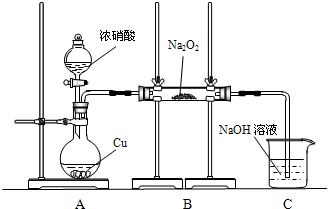
��ͼʾװ�ý���ʵ�飮
��ͨ��NO2��Na2O2��Ӧ��ȫ��Ȼ��Բ������еĹ������ʽ��м��飮
[ʵ�����]ʵ�������Na2O2��NO2�������������Ӧ��
��1��Na2O2��NO2�ķ�Ӧ�У���������______��
��2��װ��A�з�Ӧ�����ӷ���ʽ��______��
��3��װ��C��������______��
��4�������ʵ��֤��װ��B�еķ�Ӧ�����ϡ�����һ����
��5����ͬѧ��Ϊ��ֻҪֱ�ӹ۲�C�е��ܿ��Ƿ�������ð�����Ϳ����ж�B�еķ�Ӧ������һ�ּ��裬�����뷨______����ԡ����ԡ�����ԭ����______��
����һ��Na2O2+2NO2=2NaNO2+O2��
�������Na2O2+2NO2=2NaNO3
[��������]
2NaNO2+2HCl=2NaCl+NO��+NO2��+H2O
2NO2+2NaOH=NaNO2+NaNO3+H2O
[ʵ��̽��]
��ͼʾװ�ý���ʵ�飮
��ͨ��NO2��Na2O2��Ӧ��ȫ��Ȼ��Բ������еĹ������ʽ��м��飮
[ʵ�����]ʵ�������Na2O2��NO2�������������Ӧ��
��1��Na2O2��NO2�ķ�Ӧ�У���������______��
��2��װ��A�з�Ӧ�����ӷ���ʽ��______��
��3��װ��C��������______��
��4�������ʵ��֤��װ��B�еķ�Ӧ�����ϡ�����һ����
| ʵ����� | ʵ������ |
| ȡ�������еĹ������Թ��У�______�� | ______�� |

��1����Na2O2+2NO2=2NaNO3��Ӧ�У�Na2O2�е���Ԫ�ش�-1�۱�Ϊ����NaNO3�е�-2�۵���������Na2O2Ϊ��������
�ʴ�Ϊ��Na2O2��
��2��Cu+4HNO3��Ũ��=Cu��NO3��2+2NO2��+2H2O�����������ͭ��ȫ����д�����ӣ�ͭ������������ˮд��ѧʽ�����ԣ����ӷ�Ӧ����ʽΪ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
�ʴ�Ϊ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
��3��β���е�NO2���ж������壬�봦���������������������Ʒ�Ӧ2NO2+2NaOH=NaNO2+NaNO3+H2O��ֹ��Ⱦ������
�ʴ�Ϊ������β���е�NO2����ֹ��Ⱦ��
��4�����������һ���з�Ӧ��Na2O2+2NO2=2NaNO2+O2�����Թ��м����������ᣬӦ�÷���2NaNO2+2HCl=2NaCl+NO��+NO2��+H2O���к���ɫ�Ķ�������������������û����������˵�������ϡ�����һ����
�ʴ�Ϊ�����Թ��м��������������������
��5���۲�C�е��ܿ�������ð���������Ǽ���һ����һ������������һ����
�ʴ�Ϊ�����ԣ���NO2δ��ַ�Ӧ��Ҳ�ɹ۲쵽��Һ��������ð����
�ʴ�Ϊ��Na2O2��
��2��Cu+4HNO3��Ũ��=Cu��NO3��2+2NO2��+2H2O�����������ͭ��ȫ����д�����ӣ�ͭ������������ˮд��ѧʽ�����ԣ����ӷ�Ӧ����ʽΪ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
�ʴ�Ϊ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
��3��β���е�NO2���ж������壬�봦���������������������Ʒ�Ӧ2NO2+2NaOH=NaNO2+NaNO3+H2O��ֹ��Ⱦ������
�ʴ�Ϊ������β���е�NO2����ֹ��Ⱦ��
��4�����������һ���з�Ӧ��Na2O2+2NO2=2NaNO2+O2�����Թ��м����������ᣬӦ�÷���2NaNO2+2HCl=2NaCl+NO��+NO2��+H2O���к���ɫ�Ķ�������������������û����������˵�������ϡ�����һ����
�ʴ�Ϊ�����Թ��м��������������������
��5���۲�C�е��ܿ�������ð���������Ǽ���һ����һ������������һ����
�ʴ�Ϊ�����ԣ���NO2δ��ַ�Ӧ��Ҳ�ɹ۲쵽��Һ��������ð����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ