��Ŀ����
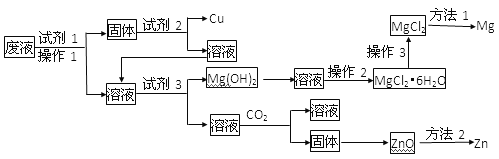
����Ŀ��ij��ҵ��Һ�к���Cu2+��Mg2+��Zn2+�����ӣ�Ϊ����������ã��ٲ��������¹���
��֪Zn(OH)2��������Al(OH)3����
�����Լ��������Լ���ѡ��
�� ���� ��п�� ��ϡHNO3 ��ϡH2SO4 ��ϡHCl ��ϡ��ˮ ��NaOH��Һ ��ʯ��ˮ
��ش��������⣺
(1)�Լ�1���Լ�2���Լ�3�ֱ������________��________��________(����)
(2)����2��_______________��______________��______________
(3)����3��________________________________
(4)�ӹ���CO2ʱ������Ӧ�����ӷ���ʽ________________________
(5)����������ʽ�����Լ�3�ܷ����Mg(OH)2��ԭ����__________________��_________________
(6)�ڽ���ұ�������з���1��_________����2��____________
���𰸡���1���ڡ��ܢݡ���(3��)
��2������Ũ������ȴ�ᾧ������(3��)
��3����HCl���������(2��)
��4��ZnO22�C + 2CO2 + 2H2O = Zn(OH)2��+ 2HCO3�C(2��)
��5��Mg2+ + 2OH�C = Mg(OH)2��(2��)Zn2++4OH�C=ZnO22�C+2H2O (2��)
��6����⡢�Ȼ�ԭ��(2��)
��������
�����������1�����ڼ����Լ�2�����ɵ���Cu����ǰ��һ������Cu2+������Լ�1ΪZn��Ϊ��ʹCu2+��Ӧ��ȫ��Zn��һ���������ʹ�������Cu��Zn�Ļ����Լ�2Ϊϡ������ϡ���ᡣΪ��ȥ��Һ�е�Mg2+��Zn2+��ֻ�ܼ���NaOH��Һ��
��2��ʹMg(OH)2����MgCl2��6H2O����ֹMg2+ˮ�⣬����Ũ������ȴ�ᾧ��������
��3��Ϊ�˷�ֹˮ�⣬��Ҫ��HCl�����м��ȣ��Ӷ��ᾧ��
��4��Zn�����NaOH��Ӧ����ZnO22-����ZnO22-+2CO2+2H2O��Zn(OH)2��+ 2HCO3-��
��5��þ����ǿ�Ӧ����������þ������п�����������ǿ�Ӧ�ò����������Ӷ�ʵ�ַ��룬����ʽΪMg2+ + 2OH�C = Mg(OH)2����Zn2+ + 4OH�C = ZnO22�C + 2H2O��
��6��ұ������þ���õ�ⷨ��ұ������пʹ���Ȼ�ԭ����

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�


