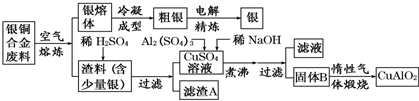
��Ŀ����
12��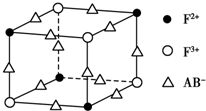 ��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2����B�ĵ縺�Դ���C������ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��F�Ļ�̬ԭ������4��δ�ɶԵ��ӣ�
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2����B�ĵ縺�Դ���C������ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��F�Ļ�̬ԭ������4��δ�ɶԵ��ӣ���1����̬��F3+��������Ų�ʽ��1s22s22p63s23p63d5��
��2��B����̬�⻯����ˮ�е��ܽ��Զ����A��C����̬�⻯�ԭ����
NH3��H2O���Ӽ���������
��3��������FD3����ɫ���塢�׳��⡢100������ʱ���������ľ��������Ƿ��Ӿ��壻������ECAB�е���������AC2��Ϊ�ȵ����壬�������ӵĵ���ʽ��
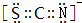 ��
����4��FD3��ECAB��Һ��ϣ��õ�������������Ѫ��ɫ��Һ��������λ��Ϊ5�������Ļ�ѧʽ��K2Fe��SCN��5��
��5��������EF[F��AB��6]��һ����ɫ���壬��ͼ��ʾ�侧����$\frac{1}{8}$��E+δ������������ɫ�����һ��������E+�ĸ���Ϊ4��
���� A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2������A��CԪ�أ�C��SԪ�أ�����ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��E��KԪ�أ�D��ԭ����������S��KԪ��֮�䣬��DΪClԪ�أ�B�ĵ縺�Դ���C����B��ԭ������С��C�����ڲ�ͬ���壬����B��NԪ�أ�Fλ�ڵ������ڣ���̬ԭ������4��δ�ɶԵ��ӣ���Χ�����Ų�Ϊ3d64s2����F��FeԪ�أ��ݴ˽��
��� �⣺A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����������������A��B��C��D��EΪ��ͬ�����Ԫ�أ�A��C��������������������Ӳ�����2������A��CԪ�أ�C��SԪ�أ�����ɫ�ܲ����۲�E����ɫ��ӦΪ��ɫ��E��KԪ�أ�D��ԭ����������S��KԪ��֮�䣬��DΪClԪ�أ�B�ĵ縺�Դ���C����B��ԭ������С��C�����ڲ�ͬ���壬����B��NԪ�أ�Fλ�ڵ������ڣ���̬ԭ������4��δ�ɶԵ��ӣ���Χ�����Ų�Ϊ3d64s2����F��FeԪ�أ�
��1����̬��Fe3+��������Ų�ʽ�ǣ�1s22s22p63s23p63d5���ʴ�Ϊ��1s22s22p63s23p63d5��
��2��B����̬�⻯��ΪNH3��A��C����̬�⻯��ֱ�ΪCH4��H2S��NH3��H2O���Ӽ�����������CH4��H2S������ˮ�����γ��������NH3��ˮ�е��ܽ��Զ����CH4��H2S��
�ʴ�Ϊ��NH3��H2O���Ӽ���������
��3��������FeCl3����ɫ���塢�׳��⡢100������ʱ�������۷е�ͣ����ڷ��Ӿ��壬������KSCN�е���������CS2��Ϊ�ȵ����壬�������ӵĵ���ʽ��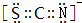 ��
��
�ʴ�Ϊ�����Ӿ��壻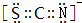 ��
��
��4��FeCl3��KSCN�õ�������������Ѫ��ɫ��Һ��������λ��Ϊ5�������Ļ�ѧʽ�ǣ�K2Fe��SCN��5��
�ʴ�Ϊ��K2Fe��SCN��5��
��5����$\frac{1}{8}$�����к���Fe2+��4��$\frac{1}{8}$=$\frac{1}{2}$����Fe3+��4��$\frac{1}{8}$=$\frac{1}{2}$����CN-��12��$\frac{1}{4}$=3�����ݻ��ϼ۴�����Ϊ0��ԭ��$\frac{1}{8}$�����к���K+��3-��$\frac{1}{2}$��2+$\frac{1}{2}$��3��=$\frac{1}{2}$����һ��������K+�ĸ���Ϊ$\frac{1}{2}$��8=4��
�ʴ�Ϊ��4��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų���������������������ʡ��ȵ����塢������������ȣ���5����ע�����þ�̯�����м��㣬�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ����0.1 mol•L-1��Na2SO3��Һ490mL������500ml����ƿ | |
| B�� | ��2�ȷݲ����͵��ռ���Һ�зֱ����һ������Na2O2��Na2O��ʹ��Һ��ǡ�ñ��ͣ�������Na2O2��Na2O�����ʵ���֮�ȵ���1��1�������¶Ȳ��䣩 | |
| C�� | �ڱ�״���£���22.4L��������1Lˮ�У��õ�1mol•L-1�İ�ˮ | |
| D�� | 10mL��������Ϊ70%���Ҵ���10mLˮϡ�ͺ��Ҵ�����������С��35% |
| A�� | �ױ�������ԭ�Ӷ�����ͬһƽ���� | |
| B�� | �������ϩ��������������Ӧ | |
| C�� | ���Ը��������Һ�����������ͼ��� | |
| D�� | ��ϩ���������������ӳɷ�Ӧ���������������ӳ� |
| A�� | MnO2��CuO��Fe�������ʵķ�ĩ���Ǻ�ɫ�ģ���ϡ����ܽ��������� | |
| B�� | ��NH4��2SO4��K2SO4��NH4Cl������Һ�����ü���NaOH��Һ�����ȵķ������ֿ� | |
| C�� | ��ȥKNO3�е�����NaCl���ɽ�������Ƴ��ȵı�����Һ����ȴ�ᾧ������ | |
| D�� | ��ʪ���KI������ֽ���Լ������������Ƿ���NO2 |
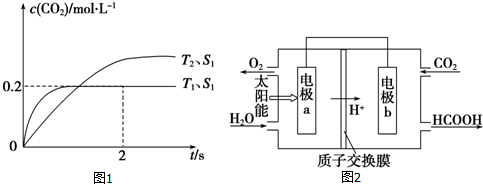
 ��1��Ԫ��M�Ƕ�����Ԫ�أ��䳣�������ں�ˮ�У����ʱ���Ϊ��������������
��1��Ԫ��M�Ƕ�����Ԫ�أ��䳣�������ں�ˮ�У����ʱ���Ϊ�������������� ��
��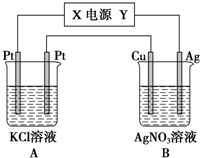 Na��Fe��Cu��Al�dz����Ľ���Ԫ�أ��밴Ҫ��ش��������⣺
Na��Fe��Cu��Al�dz����Ľ���Ԫ�أ��밴Ҫ��ش��������⣺