��Ŀ����
�����±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���д���пհף�
��1������ЩԪ���У����ʵĻ�ѧ��������õ���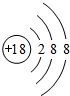
 ��
��
��2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ��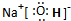
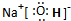 ��
��
��3������������������������Ԫ����
��4���ٺ͢���Ԫ�طֱ��γɵ�����������У��۵�ϸߵ���
��5����ѧ�ҽ���Ԫ�����ڱ��о��ϳ����ض����ʵ������ʣ�����
����ѧ�������ճ������еijԡ�����ס���С�ҽ��ϵ���У�������֬�����ۡ�������������ʳ�����ṩ��������Ҫ���ʣ��밴Ҫ��ش��������⣨�����ƣ���
��1����֬������������ˮ������ղ�����
��2������ˮ������ղ�����
��3����������һ���������ܷ���ˮ�ⷴӦ������ת��Ϊ
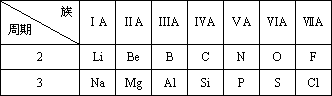
| ������ | IA | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 1 | ||||||||
| 2 | �� | |||||||
| 3 | �� | �� | �� | �� | �� |
Ar
Ar
�������Ԫ�ط��ţ���ԭ�ӽṹʾ��ͼΪ

��2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ��
HClO4
HClO4
��������ǿ�Ļ�����ĵ���ʽ��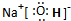
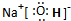
��3������������������������Ԫ����
Al
Al
�������Ԫ�ط��ţ���д���������������������Ʒ�Ӧ�Ļ�ѧ����ʽAl2O3+2OH-=2AlO2-+H2O
Al2O3+2OH-=2AlO2-+H2O
����4���ٺ͢���Ԫ�طֱ��γɵ�����������У��۵�ϸߵ���
SiO2
SiO2
������廯ѧʽ������5����ѧ�ҽ���Ԫ�����ڱ��о��ϳ����ض����ʵ������ʣ�����
������ǽ������紦
������ǽ������紦
Ѱ�Ұ뵼����ϣ�����ѧ�������ճ������еijԡ�����ס���С�ҽ��ϵ���У�������֬�����ۡ�������������ʳ�����ṩ��������Ҫ���ʣ��밴Ҫ��ش��������⣨�����ƣ���
��1����֬������������ˮ������ղ�����
��֬����
��֬����
������
����
����2������ˮ������ղ�����
������
������
����Ҫ��������ڵ���ø�������Ѿ�������ˮ�⣬��ȡ����������Һ��������������ͭ��������Һ
����������ͭ��������Һ
�����Ⱥ��ٸ���ʵ�������жϣ���Ҫ�������û����ȫˮ�⣬��ȡ����������Һ���뼸����ˮ
��ˮ
��Ӧ�۲쵽������ɫ����3����������һ���������ܷ���ˮ�ⷴӦ������ת��Ϊ
������
������
��������������Ԫ�������ڱ��е�λ�ÿ�֪����ΪC����ΪNa����ΪAl����ΪSi����ΪCl����ΪAr��
��1��ϡ�����廯ѧ�������ȶ���Arԭ�Ӻ�����3�����Ӳ㣬���ﵽ����������Ϊ2��8��8��
��2������������ǿ����������Ƶļ�����ǿ���������������������ӹ��ɣ�
��3����������������������������������Ʒ�Ӧ����ƫ��������ˮ��
��4����������ˮԭ�Ӿ��壬������̼���ڷ��Ӿ��壬�۵�ԭ�Ӿ��壾���Ӿ��壻
��5�����ڽ�����ǽ������紦��Ԫ�ؼȱ��ֽ������ֱ��ַǽ����ԣ�
����1����֬����������������ˮ��Ϊ��֬����������ͣ�
��2������ˮ������ղ����������ǣ�����������������������ͭ��������Һ����������Ƿ�ˮ�⣻
���õ������۱���ɫ����������Ƿ���ȫˮ�⣻
��3����������һ���������ܷ���ˮ�ⷴӦ������ת��Ϊ�����ᣮ
��1��ϡ�����廯ѧ�������ȶ���Arԭ�Ӻ�����3�����Ӳ㣬���ﵽ����������Ϊ2��8��8��
��2������������ǿ����������Ƶļ�����ǿ���������������������ӹ��ɣ�
��3����������������������������������Ʒ�Ӧ����ƫ��������ˮ��
��4����������ˮԭ�Ӿ��壬������̼���ڷ��Ӿ��壬�۵�ԭ�Ӿ��壾���Ӿ��壻
��5�����ڽ�����ǽ������紦��Ԫ�ؼȱ��ֽ������ֱ��ַǽ����ԣ�
����1����֬����������������ˮ��Ϊ��֬����������ͣ�
��2������ˮ������ղ����������ǣ�����������������������ͭ��������Һ����������Ƿ�ˮ�⣻
���õ������۱���ɫ����������Ƿ���ȫˮ�⣻
��3����������һ���������ܷ���ˮ�ⷴӦ������ת��Ϊ�����ᣮ
����⣺������Ԫ�������ڱ��е�λ�ÿ�֪����ΪC����ΪNa����ΪAl����ΪSi����ΪCl����ΪAr��
��1��ϡ������ԭ�������Ϊ�ȶ��ṹ��Ar��ѧ�������ȶ���Arԭ�Ӻ�����3�����Ӳ㣬���ﵽ����������Ϊ2��8��8���ṹʾ��ͼΪ ��
��
�ʴ�Ϊ��Ar�� ��
��
��2��HClO4����ǿ����������Ƶļ�����ǿ���������������������ӹ��ɣ�����ʽΪ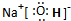 ��
��
�ʴ�Ϊ��HClO4��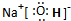 ��
��
��3����������������������������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪ��Al2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al��Al2O3+2OH-=2AlO2-+H2O��
��4����������ˮԭ�Ӿ��壬������̼���ڷ��Ӿ��壬SiO2���۵���ߣ�
�ʴ�Ϊ��SiO2��
��5�����ڽ�����ǽ������紦��Ԫ�ؼȱ��ֽ������ֱ��ַǽ����ԣ������ڴ�Ѱ�Ұ뵼����ϣ�
�ʴ�Ϊ��������ǽ������紦��
����1����֬����������������ˮ��Ϊ��֬����������ͣ�
�ʴ�Ϊ����֬���ᡢ���ͣ�
��2������ˮ������ղ����������ǣ����������£���������������ͭ��������Һ����ש��ɫ�������ɻ����������������˵�������Ѿ�ˮ�⣻��Һ�е����ˮ��������������˵��������ȫˮ�⣬
�ʴ�Ϊ�������ǣ�����������ͭ��������Һ����ˮ��
��3����������һ���������ܷ���ˮ�ⷴӦ������ת��Ϊ�����ᣮ
�ʴ�Ϊ�������ᣮ
��1��ϡ������ԭ�������Ϊ�ȶ��ṹ��Ar��ѧ�������ȶ���Arԭ�Ӻ�����3�����Ӳ㣬���ﵽ����������Ϊ2��8��8���ṹʾ��ͼΪ
 ��
���ʴ�Ϊ��Ar��
 ��
����2��HClO4����ǿ����������Ƶļ�����ǿ���������������������ӹ��ɣ�����ʽΪ
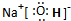 ��
���ʴ�Ϊ��HClO4��
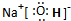 ��
����3����������������������������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪ��Al2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al��Al2O3+2OH-=2AlO2-+H2O��
��4����������ˮԭ�Ӿ��壬������̼���ڷ��Ӿ��壬SiO2���۵���ߣ�
�ʴ�Ϊ��SiO2��
��5�����ڽ�����ǽ������紦��Ԫ�ؼȱ��ֽ������ֱ��ַǽ����ԣ������ڴ�Ѱ�Ұ뵼����ϣ�
�ʴ�Ϊ��������ǽ������紦��
����1����֬����������������ˮ��Ϊ��֬����������ͣ�
�ʴ�Ϊ����֬���ᡢ���ͣ�
��2������ˮ������ղ����������ǣ����������£���������������ͭ��������Һ����ש��ɫ�������ɻ����������������˵�������Ѿ�ˮ�⣻��Һ�е����ˮ��������������˵��������ȫˮ�⣬
�ʴ�Ϊ�������ǣ�����������ͭ��������Һ����ˮ��
��3����������һ���������ܷ���ˮ�ⷴӦ������ת��Ϊ�����ᣮ
�ʴ�Ϊ�������ᣮ
���������⿼��Ԫ�����ڱ���Ԫ�������ɵ��ۺ����á����༰��֬�������ʵ����ʵȣ��ѶȲ���ּ�ڿ���ѧ���Ի���֪ʶ���������գ�ע���Ԫ�����ڱ���������գ�

��ϰ��ϵ�д�
�����Ŀ
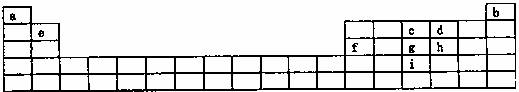
�±���Ԫ�����ڱ�����Ԫ�ص�һ���֣�
������Ԫ��W�����������������ڲ��������2����Y���ʿ��ڿ�����ȼ�գ���������������ǣ�������
| Z | ||
| W | X | Y |
| A��Y������������ˮ������ǿ�� |
| B��W������������������ռ���Һ |
| C��Z��������ֻ��һ�� |
| D��X�����������+5�� |
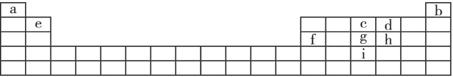
�±���Ԫ�����ڵ�һ���֣�����������ĸ�ֱ����ijһԪ�ء�
| a | b | ||||||||||||||||
| e | c | d | |||||||||||||||
| f | g | h | |||||||||||||||
| j | |||||||||||||||||
����ݱ�������Ԫ�أ��ش��������⣺
��1����̬ԭ�ӵļ۵��Ӳ��У�δ�ɶԵ���������Ԫ���� ����д������ĸ����д����۵��ӵĹ����ʾʽ ������Ԫ�ص�ԭ����aԪ�ص�ԭ���γɼķ���ʱ����Ԫ�ص�ԭ����sp3��ʽ�ӻ������γɼ��ӵ����幹��Ϊ ���÷���Ϊ ���ӣ�����ԡ��Ǽ��ԡ�����
��2��д��jԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ ��d��g��j�ֱ���a�γɻ�����ƶ����γɵĻ�����е��ɸߵ��͵�����˳��Ϊ ���ѧʽ����
��3������������ᡢ�����������������Ҫ��Ԫ���� ����д������ĸ����e��f���ʼ�ef�Ͻ���Ӳ���ɴ�С��˳��Ϊ ����д���ƣ�