��Ŀ����
�±���Ԫ�����ڵ�һ���֣�����������ĸ�ֱ����ijһԪ�ء�
| a | b | ||||||||||||||||
| e | c | d | |||||||||||||||
| f | g | h | |||||||||||||||
| j | |||||||||||||||||
����ݱ�������Ԫ�أ��ش��������⣺
��1����̬ԭ�ӵļ۵��Ӳ��У�δ�ɶԵ���������Ԫ���� ����д������ĸ����д����۵��ӵĹ����ʾʽ ������Ԫ�ص�ԭ����aԪ�ص�ԭ���γɼķ���ʱ����Ԫ�ص�ԭ����sp3��ʽ�ӻ������γɼ��ӵ����幹��Ϊ ���÷���Ϊ ���ӣ�����ԡ��Ǽ��ԡ�����
��2��д��jԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ ��d��g��j�ֱ���a�γɻ�����ƶ����γɵĻ�����е��ɸߵ��͵�����˳��Ϊ ���ѧʽ����
��3������������ᡢ�����������������Ҫ��Ԫ���� ����д������ĸ����e��f���ʼ�ef�Ͻ���Ӳ���ɴ�С��˳��Ϊ ����д���ƣ�
��1�� c
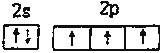
������ ����
��2��Se 1s22s22p63s23p63d104s24p4 H2O>H2Se>H2S
��3��acd þ���Ͻ�>��>þ

��ϰ��ϵ�д�
�����Ŀ
�±�ΪԪ�����ڱ���һ���������Ԫ�آ�-���ڱ��е�λ�ã��û�ѧ����ش��������⣺
��1����д���ڵ�Ԫ�ط���
��2����д���۵����������ĵ���ʽ ��
��3���ȽϢݡ��ޡ����ԭ�Ӱ뾶�ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ��
��4���ȽϢۡ��ܡ������ۺ������������ǿ������˳���ǣ�����Ļ�ѧʽ��ʾ�� ��
��5��д����Ԫ�آ�-��������������Ӧˮ�������ǿ������ǿ������֮��Ļ�ѧ��Ӧ����ʽ
��6���ߢ���Ԫ����Ƚϣ������Խ�ǿ���� �������ƣ���������֤�ý��۵�ʵ���� �����ţ���
��a�����ڿ����з����Ѿõ�������Ԫ�صĿ�״���ʷֱ������ˮ��
��b������״����С��ͬ��������Ԫ�صĵ��ʷֱ��ͬŨ�ȵ����ᷴӦ
��c������״����С��ͬ������Ԫ�صĵ��ʷֱ����ˮ���ã��������̪��Һ
��d���Ƚ�������Ԫ�ص���̬�⻯����ȶ��ԣ�
| ������ | IA | |||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | |||
��2����д���۵����������ĵ���ʽ ��
��3���ȽϢݡ��ޡ����ԭ�Ӱ뾶�ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ��
��4���ȽϢۡ��ܡ������ۺ������������ǿ������˳���ǣ�����Ļ�ѧʽ��ʾ�� ��
��5��д����Ԫ�آ�-��������������Ӧˮ�������ǿ������ǿ������֮��Ļ�ѧ��Ӧ����ʽ
��6���ߢ���Ԫ����Ƚϣ������Խ�ǿ���� �������ƣ���������֤�ý��۵�ʵ���� �����ţ���
��a�����ڿ����з����Ѿõ�������Ԫ�صĿ�״���ʷֱ������ˮ��
��b������״����С��ͬ��������Ԫ�صĵ��ʷֱ��ͬŨ�ȵ����ᷴӦ
��c������״����С��ͬ������Ԫ�صĵ��ʷֱ����ˮ���ã��������̪��Һ
��d���Ƚ�������Ԫ�ص���̬�⻯����ȶ��ԣ�
