��Ŀ����
����Ŀ��(1)��֪2 mol����ȼ������Һ̬ˮʱ�ų�572 kJ����������Ӧ����ʽ��2H2(g)��O2(g)===2H2O(l)����ش��������⣺
(1)�ٸ÷�Ӧ�������������ܺ�________(����ڡ�����С�ڡ����ڡ�)��Ӧ�������ܺ͡�
����2 mol������ȫȼ������ˮ��������ų�������________(�>������<������)572 kJ��
(2)FeS2���ղ�����SO2�����������ᡣ��֪25 �桢101 kPaʱ��
2SO2(g)��O2(g) ![]() 2SO3(g)����H1����197 kJ��mol��1
2SO3(g)����H1����197 kJ��mol��1
H2O(g)===H2O(l)����H2����44 kJ��mol��1
2SO2(g)��O2(g)��2H2O(g)===2H2SO4(l)����H3����545 kJ��mol��1
��SO3(g)��H2O(l)��Ӧ���Ȼ�ѧ����ʽ��_________________ ��
(3)��֪���з�Ӧ���Ȼ�ѧ����ʽ��
��6C(s)��5H2(g)��3N2(g)��9O2(g)===2C3H5(ONO2)3(l)����H1
��2H2(g)��O2(g)===2H2O(g)����H2
��C(s)��O2(g)===CO2(g)����H3
��Ӧ4C3H5(ONO2)3(l)===12CO2(g)��10H2O(g)��O2(g)��6N2(g)�Ħ�HΪ________��
���𰸡� С�� < SO3(g)��H2O(l)===H2SO4(l) ��H����130 kJ��mol��1 12��H3��5��H2��2��H1
��������(1)������ȼ���Ƿ��ȷ�Ӧ����˸÷�Ӧ�������������ܺ�С�ڷ�Ӧ�������ܺ͡���ˮ������������Һ̬ˮ�����������2 mol������ȫȼ������ˮ�����ų���������572 kJ��
(2)��֪��
��2SO2(g)��O2(g)![]() 2SO3(g)����H1����197 kJ��mol��1
2SO3(g)����H1����197 kJ��mol��1
��H2O(g)��H2O(l)����H2����44 kJ��mol��1
��2SO2(g)��O2(g)��2H2O(g)��2H2SO4(l)����H3����545 kJ��mol��1
����ݸ�˹���ɿ�֪���ۣ��٣��ڡ�2��/2���õ�SO3(g)��H2O(l)��Ӧ���Ȼ�ѧ����ʽ��SO3(g)��H2O(l)��H2SO4(l)����H����130 kJ��mol��1��
(3)��֪��
��6C(s)��5H2(g)��3N2(g)��9O2(g)��2C3H5(ONO2)3(l)����H1
��2H2(g)��O2(g)��2H2O(g)����H2
��C(s)��O2(g)��CO2(g)����H3
����ݸ�˹���ɿ�֪�ۡ�12+�ڡ�5���١�2���õ���Ӧ4C3H5(ONO2)3(l)��12CO2(g)��10H2O(g)��O2(g)��6N2(g)�Ħ�H��12��H3��5��H2��2��H1��

����Ŀ��ú��������úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ���̡�
(1)��ˮ����ͨ�����ȵ�̿���ɲ���ˮú������ӦΪ��
C(s)��H2O(g)![]() CO(g)��H2(g) ��H����131.3 kJ��mol��1
CO(g)��H2(g) ��H����131.3 kJ��mol��1
��ʹ��ѧ��Ӧ���ʼӿ�Ĵ�ʩ��________(�����)��
������C�����ʵ��� �����߷�Ӧ�¶�
����ʱ����CO��H2ת��ΪCH3OH ���ܱն��������г���CO(g)
(2)����ͬ����CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2 L�ĺ����ܱ������У����з�ӦCO(g)��H2O(g)CO2(g)��H2(g)���õ������������ݣ�
ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
H2O | CO | H2 | CO | |||
1 | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
2 | 900 | 2 | 4 | 0.8 | 3.2 | 3 |
��ʵ��1����v(CO2)��ʾ�Ļ�ѧ��Ӧ����Ϊ________��
�ڸ÷�Ӧ���淴ӦΪ________(������š�)�ȷ�Ӧ��
(3)��һ�ݻ�Ϊ2 L���ܱ������ڼ���2 mol��CO��6 mol��H2����һ�������·������·�Ӧ��CO(g)��2H2(g) ![]() CH3OH(g) ��H<0���÷�Ӧ���淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
CH3OH(g) ��H<0���÷�Ӧ���淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
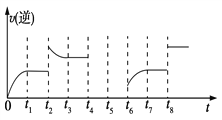
����ͼ��֪��Ӧ��t1��t3��t7ʱ���ﵽ��ƽ�⣬����t2��t8ʱ���ı������������ж�t8ʱ�ı������������________��
����t4ʱ��ѹ��t5ʱ�ﵽƽ�⣬t6ʱ����Ӧ���Ũ�ȣ�����ͼ�л���t4��t6ʱ�淴Ӧ������ʱ��Ĺ�ϵ���ߡ�________

