��Ŀ����
 ��ħ�����������������յ���Ⱦ���䷢��ԭ��������H2O2��������������������������������ݸ�ӫ�����ʺ⣮�������
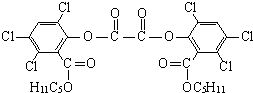
��ħ�����������������յ���Ⱦ���䷢��ԭ��������H2O2��������������������������������ݸ�ӫ�����ʺ⣮���������CPPO���ṹ��ʽ����ͼ��ʾ����ش��������⣺
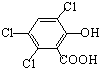
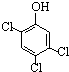
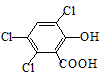
��1�����ᣨ�Ҷ��ᣩ�Ľṹ��ʽΪ
��2������CPPO����˵����ȷ����
A����������Ƿ��㻯����
B��1mol�������������4mol NaOH��Ӧ
C��1mol��������ڲ�����������6mol H2�ӳ�
D����������ķ���ʽΪC26H23O8Cl6
��3�����ᡢ�촼�ͷ��㻯����M ��
 ���ڴ������ʵ������¿�������CPPO����
���ڴ������ʵ������¿�������CPPO����������CPPO�ķ�Ӧ����Ϊ
���촼�Ĵ����ͬ���칹����
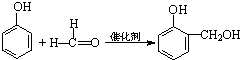
�۷��㻯����M����������N�����õ�����֪���Ӻͼ�ȩ���Է�����Ӧ��
 ��
�� �루CH3��2C=CH2Ҳ���Է������Ƶķ�Ӧ����N����N�ṹ��ʽΪ
�루CH3��2C=CH2Ҳ���Է������Ƶķ�Ӧ����N����N�ṹ��ʽΪ��������1�����ᣨ�Ҷ��ᣩ��ֻ��2��C����2��COOH��
��2��CPPO�к�-Cl��-COOC-����ϱ���±�������������ʷ�����
��3���ٲ��ᡢ�촼�ͷ��㻯����M �� ���ڴ������ʵ������¿�������CPPO��Ϊ������Ӧ��
���ڴ������ʵ������¿�������CPPO��Ϊ������Ӧ��
���촼�Ĵ����ͬ���칹��ɿ���-OHȡ�������е�1��H��������CH3��CH2��3CH3��CH3CH2��CH3��CH2CH3��C��CH3��4���Դ˷���һȡ���
1-�촼��O2������ȩΪ��������Ӧ��
������Ϣ��֪����-OH����λ�ױ�ȡ������ ��-OH����λ��ȡ������N��N��������M������
��-OH����λ��ȡ������N��N��������M������ �루CH3��2C=CH2�����ӳɵķ�Ӧ����N��
�루CH3��2C=CH2�����ӳɵķ�Ӧ����N��
��2��CPPO�к�-Cl��-COOC-����ϱ���±�������������ʷ�����
��3���ٲ��ᡢ�촼�ͷ��㻯����M ��
 ���ڴ������ʵ������¿�������CPPO��Ϊ������Ӧ��
���ڴ������ʵ������¿�������CPPO��Ϊ������Ӧ�����촼�Ĵ����ͬ���칹��ɿ���-OHȡ�������е�1��H��������CH3��CH2��3CH3��CH3CH2��CH3��CH2CH3��C��CH3��4���Դ˷���һȡ���
1-�촼��O2������ȩΪ��������Ӧ��
������Ϣ��֪����-OH����λ�ױ�ȡ������
 ��-OH����λ��ȡ������N��N��������M������
��-OH����λ��ȡ������N��N��������M������ �루CH3��2C=CH2�����ӳɵķ�Ӧ����N��
�루CH3��2C=CH2�����ӳɵķ�Ӧ����N������⣺��1�����ᣨ�Ҷ��ᣩ��ֻ��2��C����2��COOH���ṹ��ʽΪHOOC-COOH����HOOCCOOH��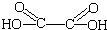 ����
����
�ʴ�Ϊ��HOOC-COOH����HOOCCOOH��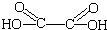 ����
����
��2��CPPO�к�-Cl��-COOC-���Һ��б�����
A����������к��������Ƿ��㻯�����A��ȷ��
B����-COOC����ˮ�������Ȼ��ͱ��ӽṹ����NaOH��Ӧ����1mol�������������6mol NaOH��Ӧ����B����
C����2�����������������ӳɷ�Ӧ����1mol��������ڲ�����������6mol H2�ӳɣ���C��ȷ��
D����������ķ���ʽΪC26H24O8Cl6����D����
�ʴ�Ϊ��AC��
��3���ٲ��ᡢ�촼�ͷ��㻯����M �� ���ڴ������ʵ������¿�������CPPO��Ϊ������Ӧ������ȡ����Ӧ��
���ڴ������ʵ������¿�������CPPO��Ϊ������Ӧ������ȡ����Ӧ��
�ʴ�Ϊ��ȡ����Ӧ����������Ӧ����
���촼�Ĵ����ͬ���칹��ɿ���-OHȡ�������е�1��H��������CH3��CH2��3CH3��CH3CH2��CH3��CH2CH3��C��CH3��4��1��H��ȡ���IJ���ֱ���3��4��1�֣������촼��3+4+1=8�֣�
1-�촼��O2������ȩΪ��������Ӧ���÷�ӦΪ2CH3��CH2��3CH2OH+O2
2CH3��CH2��3CHO+2H2O��
�ʴ�Ϊ��8��2CH3��CH2��3CH2OH+O2
2CH3��CH2��3CHO+2H2O��
������Ϣ��֪����-OH����λ�ױ�ȡ������ ��-OH����λ��ȡ������N��N��������M������
��-OH����λ��ȡ������N��N��������M������ �루CH3��2C=CH2�����ӳɵķ�Ӧ����N����NΪ
�루CH3��2C=CH2�����ӳɵķ�Ӧ����N����NΪ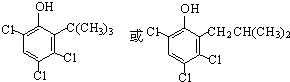 ��
��
�ʴ�Ϊ�� ��
��
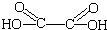 ����
�����ʴ�Ϊ��HOOC-COOH����HOOCCOOH��
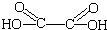 ����
������2��CPPO�к�-Cl��-COOC-���Һ��б�����
A����������к��������Ƿ��㻯�����A��ȷ��
B����-COOC����ˮ�������Ȼ��ͱ��ӽṹ����NaOH��Ӧ����1mol�������������6mol NaOH��Ӧ����B����
C����2�����������������ӳɷ�Ӧ����1mol��������ڲ�����������6mol H2�ӳɣ���C��ȷ��
D����������ķ���ʽΪC26H24O8Cl6����D����
�ʴ�Ϊ��AC��
��3���ٲ��ᡢ�촼�ͷ��㻯����M ��
 ���ڴ������ʵ������¿�������CPPO��Ϊ������Ӧ������ȡ����Ӧ��
���ڴ������ʵ������¿�������CPPO��Ϊ������Ӧ������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ����������Ӧ����
���촼�Ĵ����ͬ���칹��ɿ���-OHȡ�������е�1��H��������CH3��CH2��3CH3��CH3CH2��CH3��CH2CH3��C��CH3��4��1��H��ȡ���IJ���ֱ���3��4��1�֣������촼��3+4+1=8�֣�
1-�촼��O2������ȩΪ��������Ӧ���÷�ӦΪ2CH3��CH2��3CH2OH+O2
| Cu |
| �� |
�ʴ�Ϊ��8��2CH3��CH2��3CH2OH+O2
| Cu |
| �� |
������Ϣ��֪����-OH����λ�ױ�ȡ������
 ��-OH����λ��ȡ������N��N��������M������
��-OH����λ��ȡ������N��N��������M������ �루CH3��2C=CH2�����ӳɵķ�Ӧ����N����NΪ
�루CH3��2C=CH2�����ӳɵķ�Ӧ����N����NΪ ��
���ʴ�Ϊ��
 ��
�������������Ժϳɵ��л������ṹ�����ʣ�Ϊ��Ƶ���㣬���սṹ�й����������ʵĹ�ϵΪ���Ĺؼ�������ϩ�������������л������ʵĿ��飬ע��ͬ���칹�����д���л���Ӧ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��﮻�ʯ����Ҫ�ɷ���Li2O��Al2O3��4SiO2��������FeO��CaO��MgO�ȡ�
��﮻�ʯ����Ҫ�ɷ���Li2O��Al2O3��4SiO2��������FeO��CaO��MgO�ȡ�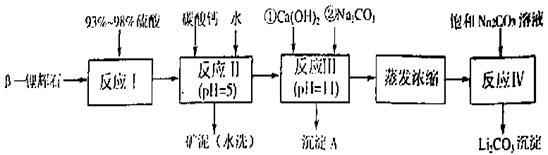
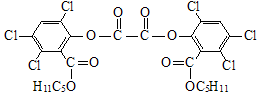
 ���ڴ������ʵ������¿�������CPPO����
���ڴ������ʵ������¿�������CPPO���� 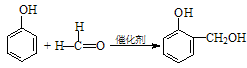 �����Ƶ�
�����Ƶ�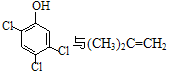 Ҳ���Է�����Ӧ����N����N�ṹ��ʽΪ_______________ ��д�����е�һ�֣���
Ҳ���Է�����Ӧ����N����N�ṹ��ʽΪ_______________ ��д�����е�һ�֣���