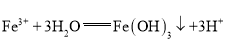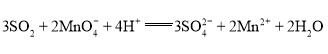��Ŀ����
����Ŀ�����ܵĴ洢������Ӧ�õ���Ҫƿ����Ŀǰ�����û������о�����Ҫ��������У���λ�⻯��������廯���̼�ʲ��ϡ������⻯��ȡ�
(1)Ti(BH4)2��һ�ֹ���Ԫ�����⻯�ﴢ����ϡ�
��Ti2+��̬�ĵ����Ų�ʽ�ɱ�ʾΪ__________________��
��BH4-�Ŀռ乹����________________(����������)��
(2)Һ���Ǹ������ʣ������ܵ��������壬����N2+3H2![]() 2NH3ʵ�ִ�������⡣
2NH3ʵ�ִ�������⡣
����������ʽ�漰�����������۵��ɵ͵��ߵ�˳����__________________��
������˵����ȷ����________(����ĸ)��
a.NH3������Nԭ�Ӳ���sp3�ӻ�
b.��ͬѹǿʱ��NH3�е��PH3��
c.[Cu(NH3)4]2���У�Nԭ������λԭ��
d.CN-�ĵ���ʽΪ![]()
(3)Ca��C60���ɵ�Ca32C60�ܴ�������H2���ӡ�
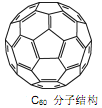
��C60���������ڱ���CS2��˵��C60��________����(���������������Ǽ�����)��
��1��C60�����У�����������ĿΪ________����
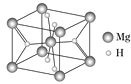
(4)MgH2�ǽ����⻯�ﴢ����ϣ��侧���ṹ��ͼ��ʾ����֪�þ�����ܶ�Ϊa g��cm-3���������Ϊ____cm3[��a��NA��ʾ(NA��ʾ�����ӵ�����)]��
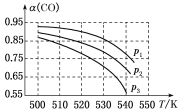
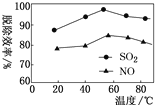
���𰸡�1s22s22p63s23p63d2(��[Ar]3d2) �������� H2< N2< NH3 abcd �Ǽ��� 90 ![]()
��������
(1)��Ti��22��Ԫ�أ�Tiԭ��ʧȥ�����2�������γ�Ti2+��Ȼ����ݹ���ԭ����д��̬�ĵ����Ų�ʽ��
�ڸ��ݼ۲���ӶԻ��������ж����ӿռ乹�ͣ�
(2)�ٸ������ʵķ��Ӽ��������ͷ���֮���Ƿ�����������жϣ�
��a.���ݼ۲���ӶԻ�������ȷ���ӻ���ʽ��
b.ͬһ����Ԫ�ص��⻯���У�����������⻯��е�ϸߣ�
c.�ṩ�µ��ӶԵ�ԭ������ԭ�ӣ�
d.CN-�Ľṹ�͵����������ƣ����ݵ������ӵĵ���ʽ�жϣ�
(3)�ٸ�����������ԭ��ȷ�����ӵļ��ԣ�
�����þ�̯�����㣻
(4)���þ�̯������þ�����þ����ԭ�Ӹ������ٸ���V=![]() ���м��㡣
���м��㡣
��Ti��22��Ԫ�أ����ݹ���ԭ����֪��̬Tiԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d24s2��Tiԭ��ʧȥ�����2�������γ�Ti2+����Ti2+��̬�ĵ����Ų�ʽ�ɱ�ʾΪ1s22s22p63s23p63d2 (��дΪ[Ar]3d2)��
��BH4-��Bԭ�Ӽ۲���Ӷ���Ϊ4+![]() =4���Ҳ����йµ��Ӷԣ�����BH4-�Ŀռ乹�������������ͣ�
=4���Ҳ����йµ��Ӷԣ�����BH4-�Ŀռ乹�������������ͣ�
(2)���ڸ÷�Ӧ���漰��������N2��H2��NH3��NH3����֮������������N2��H2����֮��ֻ���ڷ��Ӽ�������������NH3���۷е��N2��H2�ĸߣ�������Է�������N2>H2�����ʵ���Է�������Խ���Ӽ���������Խ�����ʵ��۷е��Խ�ߣ������������ʵ��۵��ɵ͵��ߵ�˳����H2< N2<NH3��
��a.NH3������Nԭ�Ӻ���3�����õ��ӶԺ�һ���µ��Ӷԣ�������۲���Ӷ���4������sp3�ӻ���a��ȷ��
b.��ͬѹǿʱ�������к��������PH3�в������������NH3�е��PH3�ߣ�b��ȷ��
c.[Cu(NH3)4]2+�����У�Nԭ���ṩ�µ��Ӷԣ�����Nԭ������λԭ�ӣ�c��ȷ��
d.CN-��C��Nԭ��ͨ�����Թ��õ��ӶԽ�ϣ������ʽΪ![]() ��d��ȷ��
��d��ȷ��
�ʺ���ѡ����abcd��
(3)�ٱ���CS2���ǷǼ��Է��ӣ�������������ԭ�����ɷǼ��Է��ӹ��ɵ��������������ɷǼ��Է��ӹ��ɵ��ܼ��У�����C60�ǷǼ��Է��ӣ�
�����þ�̯��֪��ÿ��̼ԭ�Ӻ���������ĿΪ![]() ����1mol C60�����У�����������Ŀ=
����1mol C60�����У�����������Ŀ=![]() ��1mol��60��NA/mol=90NA��
��1mol��60��NA/mol=90NA��
(4)�þ�����þԭ�Ӹ���=![]() ��8+1=2�����е�Hԭ�Ӹ���=2+4��
��8+1=2�����е�Hԭ�Ӹ���=2+4��![]() =4���������V=
=4���������V=![]() =
=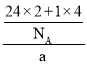 g/cm3=
g/cm3=![]() g/cm3��
g/cm3��

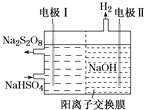
 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�����Ŀ��Na��Cu��O��Si��S��Cl�dz���������Ԫ�ء�
(1)Naλ��Ԫ�����ڱ���____���ڵ�____�壻S�Ļ�̬ԭ�Ӻ�����________��δ�ɶԵ��ӣ�Si�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ__________��
(2)����>������<����գ�
��һ������ | ���Ӱ뾶 | �۵� | ���� |
Si____S | O2-____Na+ | NaCl____Si | H2SO4____HClO4 |
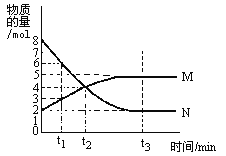
����Ŀ���������ӷ���ʽ������������Ӧʵ��������ǣ� ��
ʵ������ | ���ӷ���ʽ | |
A | ��������þ����Һ�еμ��Ȼ����Һ�������ܽ� |
|
B | ���ˮ�еμӱ����Ȼ�����Һ�õ����ɫҺ�� |
|
C | ��������ʹ���Ը��������Һ��ɫ |
|
D | ������������ϡ���� |
|
A. AB. BC. CD. D