��Ŀ����
�ۺ��Ȼ��������͡���Ч�������;�ˮ�����䵥����Һ̬��ʽ�Ȼ���[Al2(OH)nCl6��n]����ҵ�ϳ�����������Һ����ˮ���Ʊ���ʽ�Ȼ������乤���������£�
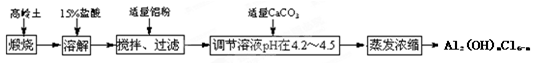
��֪����������Al2O3��25%��34%����SiO2��40%��50%����Fe2O3��0.5%��3.0%�����������ʺ�ˮ��
��Al3+������������ʽ��ȫ����ʱ����Һ��pHΪ5.2��
��������ͼ�ش��������⣺
��1��д���ܽ�����з�Ӧ�����ӷ���ʽ �� ��
��2�����������۵���ҪĿ��������������������������
��3����Һ��pH�������4.2��4.5��ԭ���� ��
��4��������Ũ�����豣���¶���90��100�棬���Ƹ��¶ȵIJ���������������������д���ù��̷�Ӧ�Ļ�ѧ����ʽ ��

��֪����������Al2O3��25%��34%����SiO2��40%��50%����Fe2O3��0.5%��3.0%�����������ʺ�ˮ��
��Al3+������������ʽ��ȫ����ʱ����Һ��pHΪ5.2��
��������ͼ�ش��������⣺
��1��д���ܽ�����з�Ӧ�����ӷ���ʽ �� ��
��2�����������۵���ҪĿ��������������������������
��3����Һ��pH�������4.2��4.5��ԭ���� ��
��4��������Ũ�����豣���¶���90��100�棬���Ƹ��¶ȵIJ���������������������д���ù��̷�Ӧ�Ļ�ѧ����ʽ ��
��1��Al2O3+6H+=2Al3++3H2O��Fe2O3+6H+=2Fe3++3H2O
��2����ȥ��Һ�е������ӣ�
��3��pH����4.5��Al3+���γ�Al(OH)3������pH����4.2��Al3+ˮ��̶Ⱥ�С�������γ�Һ̬��ʽ�Ȼ���[Al2(OH)nCl6��n]��
��4��ˮԡ���� 2AlCl3+nH2O= Al2(OH)nCl6��n +nHCl
��2����ȥ��Һ�е������ӣ�
��3��pH����4.5��Al3+���γ�Al(OH)3������pH����4.2��Al3+ˮ��̶Ⱥ�С�������γ�Һ̬��ʽ�Ȼ���[Al2(OH)nCl6��n]��
��4��ˮԡ���� 2AlCl3+nH2O= Al2(OH)nCl6��n +nHCl
�����������1����������Ҫ�ɷ�ΪAl2O3��SiO2��Fe2O3������15%�����ܽⷢ���ķ�Ӧ��Al2O3+6H+=2Al3++3H2O��Fe2O3+6H+=2Fe3++3H2O����2����Ϊ�ܽ�Һ����Fe3+���Լ����������۳�ȥ����3��Al3+��ˮ�⣬pHΪ5.2ˮ����ȫ��pH����4.5��Al3+���γ�Al(OH)3��������pH����4.2��Al3+ˮ��̶Ⱥ�С�������γ�Һ̬��ʽ�Ȼ���[Al2(OH)nCl6��n]����4�������¶���90��100�棬Ӧ��ˮԡ���ȡ�
�����������꣬��ҵ����ͼ�Ѿ���Ϊ�߿����ȵ�ϰ�⣬����ϰ����ص������ɸ��ӣ���������Ƚϼ�ԭ�������鱾�С�

��ϰ��ϵ�д�
�����Ŀ

