��Ŀ����
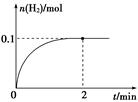
һ���¶��£���1.0L�ܱ������м���0.60molX��g����������ӦX��g��?Y��s��+2Z��g����H��0��÷�Ӧ��XŨ���뷴Ӧʱ����������±�
��1��3minʱ��Z��ʾ��ƽ����Ӧ����v��Z��= ��
��2�������÷�Ӧ�з�Ӧ���Ũ����ʱ��Ĺ��ɣ��ó��Ľ����� ���ɴ˹����Ƴ���Ӧ��6minʱ��Ӧ���Ũ��aΪ mol?L-1��
��3����Ӧ���淴Ӧ������ʱ��仯�Ĺ�ϵ��ͼ��t2ʱ�ı���ijһ���������ı������������ �� ����д������
��4��������Щ��������������Ѵ�ƽ��״̬ ������ĸ��ţ�
A���������һ��ʱ�����ܶȲ��ٱ仯
B����Ӧ��ƽ�ⳣ�����ٱ仯
C�������������ƽ����Է�����������ʱ����仯
D��Y�����ʵ������ٷ����仯
E��Z���������ʵ���X���������ʵ�2����
| ��Ӧʱ��t/min | 0 | 1 | 2 | 3 | 4 | 6 | 8 |
| c��X��/��mol?L-1�� | 0.60 | 0.42 | 0.30 | 0.21 | 0.15 | a | 0.0375 |
��2�������÷�Ӧ�з�Ӧ���Ũ����ʱ��Ĺ��ɣ��ó��Ľ�����

��3����Ӧ���淴Ӧ������ʱ��仯�Ĺ�ϵ��ͼ��t2ʱ�ı���ijһ���������ı������������
��4��������Щ��������������Ѵ�ƽ��״̬
A���������һ��ʱ�����ܶȲ��ٱ仯
B����Ӧ��ƽ�ⳣ�����ٱ仯
C�������������ƽ����Է�����������ʱ����仯
D��Y�����ʵ������ٷ����仯
E��Z���������ʵ���X���������ʵ�2����
��������1������3inX��Ũ�ȱ仯�����X�ķ�Ӧ���ʣ�Ȼ����ݻ�ѧ��������ϵ�����Z�ķ�Ӧ���ʣ�
��2�����ݱ���Ũ����ʱ��ı仯��ϵ�ҳ��仯�Ĺ��ɣ����ݱ仯���ж�a����ֵ��
��3������ͼ���֪��t2ʱ�淴Ӧ�����������ﵽƽ��ʱ�淴Ӧ���ʴ���ԭƽ��״̬ʱ�ķ�Ӧ���ʣ�
��4�����ݴﵽ��ѧƽ��״̬ʱ�����淴Ӧ������ȣ�����ֵ�Ũ�Ȳ�������жϣ�
��2�����ݱ���Ũ����ʱ��ı仯��ϵ�ҳ��仯�Ĺ��ɣ����ݱ仯���ж�a����ֵ��
��3������ͼ���֪��t2ʱ�淴Ӧ�����������ﵽƽ��ʱ�淴Ӧ���ʴ���ԭƽ��״̬ʱ�ķ�Ӧ���ʣ�
��4�����ݴﵽ��ѧƽ��״̬ʱ�����淴Ӧ������ȣ�����ֵ�Ũ�Ȳ�������жϣ�
����⣺��1��3minʱX��Ũ�ȱ仯Ϊ����0.60-0.21��mol/L=0.39mol/L��v��X��=
=0.13mol/��L?min�������ݻ�ѧ�������뷴Ӧ���ʳ����ȹ�ϵ��v��Z��=2v��X��=0.13mol/��L?min����2=0.26mol/��L?min����
�ʴ�Ϊ��0.26mol/��L?min����
��2���ӱ��е����ݿ�֪��X��Ũ�ȱ仯�Ĺ���Ϊÿ���2min��X��Ũ��Ϊԭ����һ�룬���ݴ˹��ɿ�֪��aΪ0.15��һ�룬��0.075��
�ʴ�Ϊ��ÿ���2min��X��Ũ��Ϊԭ����һ�룻0.075��
��3����ͼ�����֪������t2ʱ�淴Ӧ�������������Ҵﵽƽ��ʱ�淴Ӧ���ʴ���ԭƽ��״̬ʱ�ķ�Ӧ���ʣ�˵���ﵽƽ��ʱ����Ϊ��Ũ�ȴ���ԭƽ���Ũ�ȣ�����Ӱ�췴Ӧ���ʵ����ط��������Լ���������Z���淴Ӧ���ʻ��������ﵽ�µ�ƽ��ʱZ��Ũ�ȱ�ԭƽ���Ũ�ȴ�Ӧ���ʲ���Ҳ������С���������������ѹǿ�����淴Ӧ���ʶ������Ҵﵽƽ��ʱ���淴Ӧ���ʶ�����ԭƽ������ʣ�
�ʴ�Ϊ������Z��������ϵ��ѹǿ��
��4��A���������һ��ʱ�����ܶȲ��ٱ仯������YΪ���壬��Ӧ����ʽ�����������������ȣ��������ݻ��̶��������ܶȹ�ʽ��֪���ܶ��ܶȲ��䣬˵�����淴Ӧ������ȣ�����ֵ�Ũ�Ȳ��䣬�ﵽ��ƽ��״̬����A��ȷ��
B����Ӧ��ƽ�ⳣ�����ٱ仯����ѧƽ�ⳣ�����¶��йأ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣬���Ի�ѧƽ�ⳣ�����ж��Ƿ�ﵽ��ƽ��״̬����B����
C�����ڷ�Ӧ�����������������ȣ���������ʵ�������ȣ������ƽ���������Ǹ��仯�������������������ƽ����Է�����������ʱ����仯��˵�����淴Ӧ������ȣ��ﵽ��ƽ��״̬����C��ȷ��
D��Y�����ʵ������ٷ����仯��Y�����ʵ������䣬˵�����淴Ӧ������ȣ��ﵽ��ƽ��״̬����D��ȷ��
E��Z���������ʵ���X���������ʵ�2������ʾ��������Ӧ���ʣ����ж����淴Ӧ�����Ƿ���ȣ���E����
��ѡACD��
| 0.39mol?L-1 |
| 3min |
�ʴ�Ϊ��0.26mol/��L?min����
��2���ӱ��е����ݿ�֪��X��Ũ�ȱ仯�Ĺ���Ϊÿ���2min��X��Ũ��Ϊԭ����һ�룬���ݴ˹��ɿ�֪��aΪ0.15��һ�룬��0.075��
�ʴ�Ϊ��ÿ���2min��X��Ũ��Ϊԭ����һ�룻0.075��
��3����ͼ�����֪������t2ʱ�淴Ӧ�������������Ҵﵽƽ��ʱ�淴Ӧ���ʴ���ԭƽ��״̬ʱ�ķ�Ӧ���ʣ�˵���ﵽƽ��ʱ����Ϊ��Ũ�ȴ���ԭƽ���Ũ�ȣ�����Ӱ�췴Ӧ���ʵ����ط��������Լ���������Z���淴Ӧ���ʻ��������ﵽ�µ�ƽ��ʱZ��Ũ�ȱ�ԭƽ���Ũ�ȴ�Ӧ���ʲ���Ҳ������С���������������ѹǿ�����淴Ӧ���ʶ������Ҵﵽƽ��ʱ���淴Ӧ���ʶ�����ԭƽ������ʣ�
�ʴ�Ϊ������Z��������ϵ��ѹǿ��
��4��A���������һ��ʱ�����ܶȲ��ٱ仯������YΪ���壬��Ӧ����ʽ�����������������ȣ��������ݻ��̶��������ܶȹ�ʽ��֪���ܶ��ܶȲ��䣬˵�����淴Ӧ������ȣ�����ֵ�Ũ�Ȳ��䣬�ﵽ��ƽ��״̬����A��ȷ��
B����Ӧ��ƽ�ⳣ�����ٱ仯����ѧƽ�ⳣ�����¶��йأ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣬���Ի�ѧƽ�ⳣ�����ж��Ƿ�ﵽ��ƽ��״̬����B����
C�����ڷ�Ӧ�����������������ȣ���������ʵ�������ȣ������ƽ���������Ǹ��仯�������������������ƽ����Է�����������ʱ����仯��˵�����淴Ӧ������ȣ��ﵽ��ƽ��״̬����C��ȷ��
D��Y�����ʵ������ٷ����仯��Y�����ʵ������䣬˵�����淴Ӧ������ȣ��ﵽ��ƽ��״̬����D��ȷ��
E��Z���������ʵ���X���������ʵ�2������ʾ��������Ӧ���ʣ����ж����淴Ӧ�����Ƿ���ȣ���E����
��ѡACD��
���������⿼���˻�ѧ��Ӧ�����뻯ѧ�������Ĺ�ϵ����ѧƽ��״̬���жϡ���ѧƽ���Ӱ�����ص�֪ʶ���Ѷ��еȣ����Ը�����ѧ��֪ʶ��ɣ�

��ϰ��ϵ�д�
�����Ŀ
��14�֣���Դ������������������Դ���õ��ǵ����������Ż��⡣�������ѧ��ѧ֪ʶ�ش��������⣺
��1�������ϰ�װ��ת��������ʹ����β���е���Ҫ��Ⱦ�CO��NOx��̼�⻯����������Ӧ�����������ʣ���������β����Ⱦ��
��֪��N2(g) + O2(g)��2NO(g) ��H��+180.5 kJ �� mol��1��
2C(s)+ O2(g)��2CO(g) ��H����221.0 kJ �� mol��1��
C(s)+ O2(g)��CO2(g) ��H����393.5 kJ �� mol��1
��β��ת����Ӧ2NO(g) +2CO(g)��N2(g)+2CO2(g)�ġ�H��________________��
��2������β�������Ƕ�CO�ĺ�����������ȼ�ϵ��Ϊ����ԭ������װ������ͼ��ʾ���õ���е����Ϊ�����ƣ������ƣ�����O2�������ڹ�������������ƶ���
����˵������ȷ����_____________(����ĸ���)��
| A�������ĵ缫��ӦʽΪ��CO + O2���D2e����CO2 |
| B������ʱ�����ɵ缫aͨ������������缫b |
| C������ʱ�缫b��������O2���ɵ缫aͨ�����������缫bǨ�� |
| D����������ͨ���ĵ���Խ��β����CO�ĺ���Խ�� |
��4��ú�ļ��Һ������ת��ΪCO��H2�����ڴ��������ºϳɼ״�������һ���¶��£���1 L�ܱ������м���CO��H2��������ӦCO(g)+2H2(g)
 CH3OH(g)����10 min��Ӧ�ﵽƽ��ʱ��ø���ֵ�Ũ�����£�
CH3OH(g)����10 min��Ӧ�ﵽƽ��ʱ��ø���ֵ�Ũ�����£�| �� �� | CO | H2 | CH3OH |
| Ũ��/(mol��L��1) | 1.2 | 1.0 | 0.6 |
�ڸ�ʱ���ڷ�Ӧ����v(H2)��_________________��
��ƽ��ʱCO��ת����Ϊ_________________(����1λС��)��
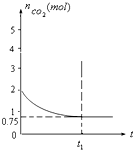 һ���¶��£���1���ĺ��������м���2moL̼��2moLCO2����ӦΪ��
һ���¶��£���1���ĺ��������м���2moL̼��2moLCO2����ӦΪ�� H2��I2
H2��I2