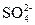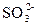��Ŀ����
��11�֣���һƿ��ɫ������ܺ���H2S��CO2��HBr��SO2��Cl2�е�һ�ֻ��֣�����ͨ��ϡ��ˮ�е���ɫ����Һ������Һ�ֳ����ݣ���һ���м�����ϡHNO3�ữ��AgNO3�����ɰ�ɫ����������һ���м����������ữ��BaCl2��Һ��Ҳ���ɰ�ɫ������
��1��ԭ�����п϶�����____________________________�����ܺ���
____________________________��?
��2���Կ��ܺ��еijɷ֣��������һ������ʵ�鷽����������˵���ڴ˷����У�����ͨ���ĸ�װ�õ����á�
��1��ԭ�����п϶�����____________________________�����ܺ���
____________________________��?
��2���Կ��ܺ��еijɷ֣��������һ������ʵ�鷽����������˵���ڴ˷����У�����ͨ���ĸ�װ�õ����á�
��1��SO2��CO2
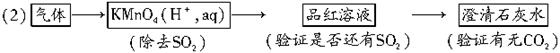
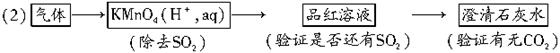
��1����Ϊ����Ϊ��ɫ���壬���Բ���Cl2��������ͨ��ϡ��ˮ�е���ɫ����Һ��֤������H2S��HBr����SO2����Ϊ�������H2S���������з�Ӧ��
H2S+Cl2====2HCl+S��
���ɵ�S��ʹ��Һ����ǣ��������HBr����ᷢ�����з�Ӧ��
2HBr+Cl2====2HCl+Br2
���ɵ�Br2��ʹ��Һ�Ի�ɫ�����ɫ�����������SO2���Ͳ��ᷢ�����з�Ӧ��
SO2+Cl2+2H2O====2HCl+H2SO4
���������з�Ӧ���Ͳ����ȥ��ˮ�е�Cl2��������Һ�Ͳ�����ɫ������dz��?��ɫ��
��������ͨ����ˮ��������ҺΪϡHCl��aq����ϡH2SO4��aq���Ļ����Һ������AgNO3��aq������AgCl������
Ag++Cl��====AgCl��
����BaCl2��aq������BaSO4������
Ba2++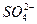 ====BaSO4
====BaSO4
�ɴ�Ҳ��֤��ԭ����϶�����SO2��CO2���ܺ��У�Ҳ���ܲ����С�
��2������֤CO2���ɳ�ȥSO2������֤����SO2��ʣ������ͨ������ʯ��ˮ��ʯ��ˮ����ǣ�֤����CO2��ʯ��ˮ������ǣ�֤������CO2��
H2S+Cl2====2HCl+S��
���ɵ�S��ʹ��Һ����ǣ��������HBr����ᷢ�����з�Ӧ��
2HBr+Cl2====2HCl+Br2
���ɵ�Br2��ʹ��Һ�Ի�ɫ�����ɫ�����������SO2���Ͳ��ᷢ�����з�Ӧ��
SO2+Cl2+2H2O====2HCl+H2SO4
���������з�Ӧ���Ͳ����ȥ��ˮ�е�Cl2��������Һ�Ͳ�����ɫ������dz��?��ɫ��
��������ͨ����ˮ��������ҺΪϡHCl��aq����ϡH2SO4��aq���Ļ����Һ������AgNO3��aq������AgCl������
Ag++Cl��====AgCl��
����BaCl2��aq������BaSO4������
Ba2++
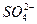 ====BaSO4
====BaSO4�ɴ�Ҳ��֤��ԭ����϶�����SO2��CO2���ܺ��У�Ҳ���ܲ����С�
��2������֤CO2���ɳ�ȥSO2������֤����SO2��ʣ������ͨ������ʯ��ˮ��ʯ��ˮ����ǣ�֤����CO2��ʯ��ˮ������ǣ�֤������CO2��

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ