��Ŀ����
����Ŀ��������ͬѧ����Ϥ�����ʣ�
��O2��H2O2��MgCl2��H2SO4��Na2CO3��NH4Cl��CO2��Ne��Na2O2��NaOH
(1)��Щ�����У����ڹ��ۻ��������________(����ţ���ͬ)��ֻ�������Ӽ�����________��������ѧ������________��
(2)д���������ʵĽṹʽ��
��O2________����H2O2_______��
(3)д���������ʵĵ���ʽ
��Na2O2__________����CO2__________����NH4Cl____________��
(4)�õ���ʽ��ʾ��MgCl2���γɹ��̣�________________________________________��
���𰸡��ڢܢ� �� �� O=O H-O-O-H ![]()
![]()
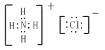
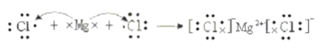
��������
ֻ���й��ۼ��Ļ�����Ļ�����Ϊ���ۻ�����������Ӽ��Ļ�����Ϊ���ӻ����ϡ������Ϊ��ԭ�ӷ��Ӳ������ۼ���
��O2ֻ���зǼ��Թ��ۼ������ڵ��ʣ�
��H2O2���м��ԺͷǼ��Թ��ۼ������ڹ��ۻ����
��MgCl2ֻ�������Ӽ����������ӻ����
��H2SO4ֻ���й��ۼ������ڹ��ۻ����
��Na2CO3�Ⱥ������Ӽ����ֺ��й��ۼ����������ӻ����
��NH4Cl�Ⱥ������Ӽ����ֺ��м��Թ��ۼ����������ӻ����
��CO2ֻ���м��Թ��ۼ������ڹ��ۻ����
��Ne��ϡ�����嵥�ʣ�ϡ�������ǵ�ԭ�ӷ��ӣ�������ѧ����
��Na2O2�Ⱥ������Ӽ����ֺ��зǼ��Թ��ۼ����������ӻ����
��NaOH�Ⱥ������Ӽ����ֺ��м��Թ��ۼ����������ӻ����
(1)��Щ�����У����ڹ��ۻ���������ڢܢ���ֻ�������Ӽ���������������ѧ�����Ǣࣻ
(2)��O2ֻ���зǼ��Թ��ۼ��ĵ��ʣ���ԭ��֮���γ��������õ��Ӷԣ��ṹʽΪO=O��
��H2O2�Ǻ��м��ԺͷǼ��Թ��ۼ��Ĺ��ۻ������ԭ�Ӽ��γɷǼ��Թ��ۼ�����ԭ������ԭ��֮���γɼ��Թ��ۼ����ṹʽΪH-O-O-H��
(3)��Na2O2��Na+��O22-�γ����Ӽ�����ԭ��֮���γɷǼ��Թ��ۼ�������ʽΪ![]() ��
��
��CO2�ǹ��ۻ����̼ԭ������ԭ��֮���γ�2�����Թ��ۼ�������ʽΪ![]() ��
��
��NH4Cl�����ӻ������笠����Ӻ������ӹ��ɣ�笠������ڲ���ԭ������ԭ���γɼ��Թ��ۼ�������ʽΪ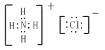 ��
��
(4)��MgCl2���ӻ�����������Ӻ�þ���ӹ��ɣ�þԭ��ʧȥ���ӣ���ԭ�ӵõ����ӣ����γɹ��̣�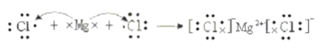 ��
��

����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�~���ڱ��е�λ�ã��û�ѧ����ش��������⡣
�� ���� | IA | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | ||||
(1)Ԫ�آڵ�ԭ�ӽṹʾ��ͼΪ_____��
(2)Ԫ�آۡ��ܡ��ݡ���ԭ�Ӱ뾶�ɴ�СΪ_____��
(3)����8��Ԫ���У��ǽ�������ǿ����_____��
(4)Ԫ�آڡ����γɵ���̬�⻯���ȶ���:_____��_____��Ԫ�آ١��ۡ��ݿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д�������ʽ��_____��
(5)Ԫ�آߵ�����������Ӧ��ˮ������Ԫ�آ�����������Ӧ��ˮ������Һ��Ӧ�����ӷ���ʽΪ_____��
(6)��ԭ�ӽṹ�ĽǶȽ���Ԫ�آۺ͢ߵķǽ�����ǿ����_________________________��
����Ŀ�����������LiFePO4��һ��������������ӵ�صĵ缫���ϡ�ij�����������졢﮻�ʯLiAl��SiO3��2��������Ca2+��Mg2+���Σ���̼�۵�ԭ����������������ﮡ�����Ҫ����������ͼ��

��֪��2LiAl��SiO3��2+H2SO4(Ũ)![]() Li2SO4+Al2O3��4SiO2��H2O��
Li2SO4+Al2O3��4SiO2��H2O��
�¶�/�� | 20 | 40 | 60 | 80 | |
�ܽ��(Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 | |
�ܽ��(Li2SO4)/g | 34.2 | 32.8 | 31.9 | 30.7 |
��1�����������пɷ����Al2O3����ͼ��ʾ����д�����ɳ��������ӷ���ʽ___��
![]()
��2�����������Ҫ�ɷ��ǣ�___���ѧʽ����
��3������Һ���м��뱥��Na2CO3��Һ�����˺�������ˮϴ������ԭ����___��
��4��д���ڸ�����������������﮵Ļ�ѧ����ʽ��___��
��5����������﮵���ܷ�ӦΪ��FePO4+Li![]() LiFePO4������еĹ������ʿɴ���Li+����д���õ�طŵ�ʱ��������Ӧ��___�����øõ�ص�ⱥ��ʳ��ˮ�����ص缫��Ϊ���Ե缫������������������4480mL���壨��״��������ʱ���õ������﮵�����Ϊ___��
LiFePO4������еĹ������ʿɴ���Li+����д���õ�طŵ�ʱ��������Ӧ��___�����øõ�ص�ⱥ��ʳ��ˮ�����ص缫��Ϊ���Ե缫������������������4480mL���壨��״��������ʱ���õ������﮵�����Ϊ___��








