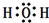��Ŀ����
��ͭ����п���õ������ӣ����β���ֱ�ʢ�У���������Һ��������ͭ��Һ������������Һ�������ձ��У���ʱ��
(1)ͭƬ�Ϸ�������Ҫ��Ӧ�ĵ缫��Ӧʽ�ǣ���________________________��
��______________________________________________________________��
��_______________________________________________________________��
(2)����Ʒ�ھ���������ĵ����绯ѧ��ʴ���أ�д���˵绯ѧ��ʴ�ĵ缫��Ӧʽ��
����____________________________________________________________��
����____________________________________________________________��
(3)������ȥ������Ĥ��þƬ����Ƭ�ô��������ĵ������ӣ�����ʢ���ռ���Һ���ձ��У���ʱ���ֵ�����ָ��ƫת���жϴ�ԭ��ص�����������д���缫��Ӧʽ���ܷ�Ӧ����ʽ��
������________���缫��Ӧʽ��_____________________________________��
������________���缫��Ӧʽ��_____________________________________��
�ܷ�Ӧ��________________________________________��
(1)��2H����2e��===H2������Cu2����2e��===Cu
��O2��2H2O��4e��===4OH��
(2)2H����2e��===H2����Fe��2e��===Fe2��
(3)Mg��6H2O��6e��===3H2����6OH����Al��2Al��8OH����6e��===2[Al(OH)4]����2Al��2NaOH��6H2O===2Na[Al(OH)4]��3H2��

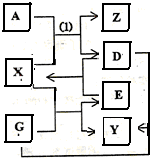 ��2011?����ģ�⣩A��D��E��G���ֵ��ʺ�X��Y��Z���ֻ����������ѧ��ѧ�е���Ҫ���ʣ�����֮������ͼ��ʾ��ת����ϵ�����³�ѹ�£�X����ɫ��ζ��Һ�壬Y�Ǻ�ɫ���壬Z��ˮ��Һ��һ�����ᣬ��Ӧ��1�������ڲ��������н��У���ش��������⣺
��2011?����ģ�⣩A��D��E��G���ֵ��ʺ�X��Y��Z���ֻ����������ѧ��ѧ�е���Ҫ���ʣ�����֮������ͼ��ʾ��ת����ϵ�����³�ѹ�£�X����ɫ��ζ��Һ�壬Y�Ǻ�ɫ���壬Z��ˮ��Һ��һ�����ᣬ��Ӧ��1�������ڲ��������н��У���ش��������⣺