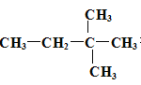
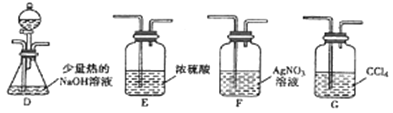
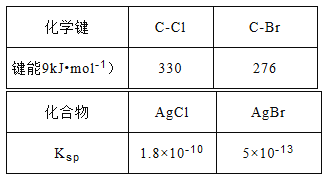
��Ŀ����
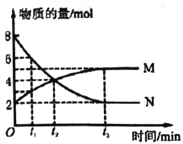
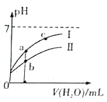
����Ŀ���������Һ�絼��Խ������Խǿ����������0.100 mol��L-1����ֱ�ζ�10.00 mLŨ�Ⱦ�Ϊ0.100 mol��L-1��NaOH��Һ�Ͷ��װ���(CH3)2NH����Һ�����װ���ˮ�е����백���ƣ�����Ksp[(CH3)2NH��]=l.6��10-4�������ô�������õζ���������Һ�ĵ絼����ͼ��ʾ������˵����ȷ����

A. ���ߢٴ����ζ����װ���Һ������
B. a����Һ�У�c[(CH3)2NH2+]>c[CH3]2NH��H2O]
C. d����Һ�У�c(H+)=c(OH-)+c[CH3]2NH��H2O]
D. b��c��e�������Һ�У�ˮ�ĵ���̶�������b��
���𰸡�C
��������A�����װ���������ʣ���Һ������Ũ�Ƚ�С�������ᷴӦ����Һ������Ũ��������Һ�ĵ�������ǿ��������ߢ��ǵζ����װ���Һ���ʴ���B�����ߢ��ǵζ�NaOH��Һ�����ߣ��ʴ���C���Ѷ��װ�������NH3������10mL���ᣬ����ǡ����ȫ��Ӧ�����������غ㣬�����c(H+)=c(OH-)+c[��CH3��2NH��H2O]������ȷ��D��b�㷴Ӧ����Һ�е������Ƕ��װ��� (CH3)2NH2Cl����Һ�Լ��ԣ����װ��ĵ�������ˮ�ĵ��룬c��ǡ����ȫ��Ӧ������ΪNaCl��e���������������ˮ�ĵ��룬��˵���̶�������c�㣬�ʴ���