��Ŀ����
����Ŀ����ˮ�й����İ���(NH3��NH4+)�ᵼ��ˮ�帻Ӫ������ij����С����NaClO����������������ˮ����֪����HClO�������Ա�NaClOǿ����NH3��NH4+���ױ��������۹��ұ�Ҫ�������İ�����ˮpHҪ������6��9��
��1��pH��1.25ʱ��NaClO����NH4+��Ӧ����N2������Ⱦ���ʣ��÷�Ӧ�����ӷ���ʽΪ________��
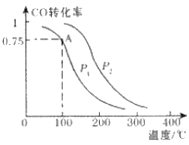
��2����ˮpH����ȥ���ʺͳ�ˮpH��Ӱ����ͼ��ʾ
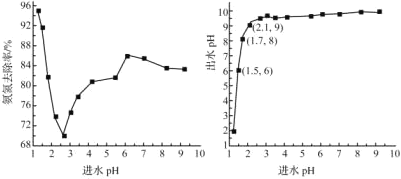
�ٽ�ˮpHΪ1.25��2.75��Χ�ڣ�����ȥ������pH����Ѹ���½���ԭ����_______
�ڽ�ˮpHΪ2.75��6.00��Χ�ڣ�����ȥ������pH���߶�������ԭ����______
�۽�ˮpHӦ������______����Ϊ�ˡ�
��3��Ϊ�о�������NaClO����������Ӱ�죬�����������䣬�����ӵ�λʱ����ͨ��������������ְ���ȥ���ʼ������䡣��ԭ�������_______(����ĸ)��
a��O2�������Ա�NaClO�� b��O2�����������ʱ�NaClO��
c��O2����Һ���ܽ�ȱȽ�С d�������е�N2������Һ��
���𰸡�3ClO����2NH4+=N2����3Cl����3H2O��2H��(��3HClO��2NH4+=N2����3Cl����3H2O��5H��) ����pH���ߣ�NaClO���������������ܽ��ͣ����°���ȥ�����½� ����pH���ߣ�������ˮ��NH3�������������ױ����� 1.50 abc
��������
(1)pH=1.25ʱ��NaClO����NH4+��Ӧ����N2������Ⱦ���ʣ�����������ӱ���ԭΪ�����Ӻ�ˮ����ϵ���غ㡢�����غ㡢ԭ���غ���ƽ��д���ӷ���ʽ��
(2)�ٽ�ˮpHΪ1.25��2.75��Χ�ڣ����HClO�������Ա�NaClOǿ������𣻢ڽ�ˮpHΪ2.75��6.00��Χ�ڽ��NH3��NH4+���ױ�����������𣻢۸���ͼ���Ϸ�ˮ�ŷű������жϣ�
(3)�����������䣬�����ӵ�λʱ����ͨ��������������ְ���ȥ���ʼ������䣬˵��O2�����������ʱ�NaClO�����ȴ�������������������Һ���ܽ�������ٵ�ԭ��
(1)pH=1.25ʱ��NaClO����NH4+��Ӧ����N2������Ⱦ���ʣ�����������ӱ���ԭΪ�����ӣ���ϵ���غ㡢�����غ㡢ԭ���غ���ƽ��д���ӷ���ʽ��3ClO����2NH4+=N2����3Cl����3H2O��2H�����ʴ�Ϊ��3ClO����2NH4+=N2����3Cl����3H2O��2H����
(2)�ٽ�ˮpHΪ1.25��2.75��Χ�ڣ�����pH���ߣ�HClO�������ͣ�NaClO�ĺ������ߣ�����HClO�������Ա�NaClOǿ��NaClO���������������ܽ��ͣ����°���ȥ�����½����ʴ�Ϊ������pH���ߣ�NaClO���������������ܽ��ͣ����°���ȥ�����½���
�ڽ�ˮpHΪ2.75��6.00��Χ�ڣ�����pH���ߣ�������ˮ�а���������������NH3��NH4+���ױ�������ʹ�ð������ױ��������ʴ�Ϊ������pH���߰�����ˮ�а����������������ױ�������
�۸���ͼʾ����Ϲ��ұ�Ҫ�������İ�����ˮpHҪ������6��9����ˮpHӦ������1.50���ң�����ȥ���ʻ�ϴ������İ�����ˮpH��6���ң������ŷű����ʴ�Ϊ��1.50��
(3)�о�������NaClO����������Ӱ�죬�����������䣬�����ӵ�λʱ����ͨ��������������ְ���ȥ���ʼ������䡣��ԭ������ǣ�O2�������Ա�NaClO����O2�����������ʱ�NaClO����O2����Һ���ܽ�ȱȽ�С���ʴ�Ϊ��abc��