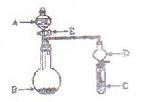
��Ŀ����
��9�֣�ʵ������Ҫ0.2mol/L��CuSO4��Һ480mL�������Ƹ���Һ��ʵ��������裺
E����ƿ���������������µߵ���ҡ�ȡ�
����д���пհף�
��1����� gCuSO4��5H2O����
��2�������������ȷ˳��Ϊ ������ţ�
��3����ʵ���õ��Ļ������������ձ�����ƽ�����롢���ӣ���ҩ�ף�
��ȱ�ٵ������� �� �� ��
��4�����������ʹ������ҺŨ��ƫ�͵��� ������ţ�
a.����ʱ���ӡ� b.û�н��������IJ�������B
c.������ˮʱ�� ���������˿̶��� d.������մ�����ʣ�����ʹ����������룩
���������˿̶��� d.������մ�����ʣ�����ʹ����������룩
e.����ƿʹǰδ�����
A����������ƽ��ȡgCuSO4��5H2O���壬�����ձ��У��� ����������ˮʹ���ܽⲢ�ָ������¡� ����������ˮʹ���ܽⲢ�ָ������¡� |
| B��������ˮϴ�ձ��Ͳ�����2��3�Σ�ÿ��ϴ��Һ��С��ע������ƿ���������� |
| C������������ƿ������ˮ����Һ���̶���1cm~2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����ʹ���̶������С� |
| D�����Ƶõ���Һͨ��������������С�ĵ���������ƿ�С� |
����д���пհף�
��1����� gCuSO4��5H2O����
��2�������������ȷ˳��Ϊ ������ţ�
��3����ʵ���õ��Ļ������������ձ�����ƽ�����롢���ӣ���ҩ�ף�
��ȱ�ٵ������� �� �� ��
��4�����������ʹ������ҺŨ��ƫ�͵��� ������ţ�
a.����ʱ���ӡ� b.û�н��������IJ�������B
c.������ˮʱ��
 ���������˿̶��� d.������մ�����ʣ�����ʹ����������룩
���������˿̶��� d.������մ�����ʣ�����ʹ����������룩e.����ƿʹǰδ�����
��

��ϰ��ϵ�д�
�����Ŀ

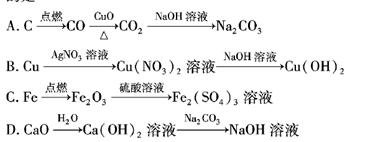
 ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������ ��
����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������ ��
 +4����ȡ����TiO2���������£�
+4����ȡ����TiO2���������£�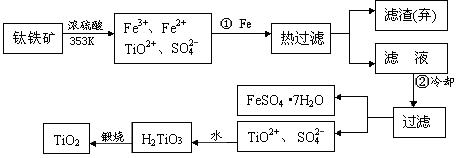
 TiCl4
TiCl4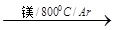 Ti
Ti 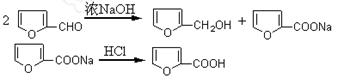
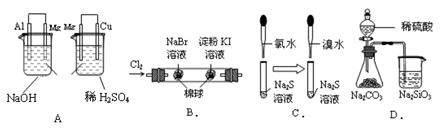
 ���������ʵ�顣��ʵ��װ������ͼ��ʾ��
���������ʵ�顣��ʵ��װ������ͼ��ʾ��