题目内容
【题目】污染物的有效去除和资源的充分利用是化学造福人类的重要课题。某研究小组利用软锰矿(主要成分为MnO2,另含有少量铁、铝、铜、镍等金属化合物)作脱硫剂,通过如下简化流程既脱除燃煤尾气中的SO2,又制得电池材料 MnO2(反应条件已省略)。
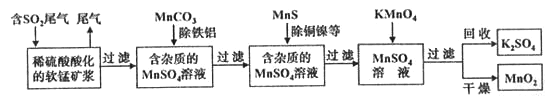
请回答下列问题:
(1)上述流程脱硫实现了____________(选填下列字母编号)。
a.废弃物的综合利用 b.白色污染的减少 c.酸雨的减少
(2)KMnO4+MnSO4→MnO2的反应中,氧化剂与还原剂的物质的量之比为____________。
(3)已知:25℃、101kPa 时,Mn(s)+O2(g)=MnO2(s) △H=-520kJ·mol-1
S(s)+O2(g)=SO2(g) △H=-297 kJ·mol-1
Mn(s)+S(s)+2O2(g)= MnSO4(s) △H=-1065 kJ·mol-1
SO2与MnO2反应生成无水MnSO4的热化学方程式为:_______________________________。
(4)MnO2可作超级电容器材料。用惰性电极电解MnSO4溶液可制得MnO2其阳极的电极反应式是__________________________________________________。
(5)MnO2是碱性锌锰电池的正极材料。碱性锌锰电池放电时,正极的电极反应式为:____________________________________________________。若以该电池为电源,以石墨作电极电解CuSO4溶液,阴极析出铜,阳极产物是___________________。
【答案】 a c 2∶3 MnO2(s)+ SO2(g)= MnSO4(s) △H=-248 kJ·mol-1 Mn2++2H2O-2e-=MnO2+4H+ MnO2+H2O+e-= MnO(OH)+OH- O2 、H2SO4
【解析】(1)白色污染主要是塑料等难降解的物质形成的,SO2能形成酸雨,因此脱硫实现了废弃物的综合利用,同时也减少了酸雨形成,即答案选ac;(2)KMnO4+MnSO4→MnO2的反应中,根据化合价变化,KMnO4→MnO2的化合价由+7降到+4降低4,+MnSO4→MnO2化合价由+2升到+4升高2,氧化剂与还原剂的物质的量之比为2:3;(3)已知:热化学方程式①Mn(s)+O2(g)=MnO2(s) △H=-520kJ/mol,②S(s)+O2(g)=SO2(g)
△H=-297kJ/mol,③Mn(s)+S(s)+2O2(g)=MnSO4(s) △H=-1065kJ/mol,则根据盖斯定律可知③-(①+③)即得到SO2与MnO2反应生成无水MnSO4的热化学方程式MnO2(s)+SO2(g) =MnSO4(s) △H=-248kJ/mol;(4)电解池中阳极失去电子发生氧化反应,则用惰性电极电解MnSO4溶液可制得MnO2,因此阳极是锰离子放电,其阳极电极反应式是Mn2++2H2O-2e-=MnO2+4H+;(5)原电池中负极失去电子,正极得到电子,因此碱性锌锰电池放电时,正极是二氧化锰得到电子,则电极反应式是MnO2+H2O+e-=MnO(OH)+OH-;以石墨作电极电解CuSO4 溶液。阴极析出铜,则阳极为阴离子氢氧根放电,生成氧气,导致氢离子浓度增大,所以阳极的产物有O2 、H2SO4。

 名师金手指领衔课时系列答案
名师金手指领衔课时系列答案【题目】常温下, 下列各组物质中,Y既能与X反应又能与Z反应的是( )
X | Y | Z | |
① | NaOH溶液 | Al(OH)3 | 稀硫酸 |
② | KOH溶液 | SiO2 | 稀盐酸 |
③ | O2 | N2 | H2 |
④ | FeCl3溶液 | Cu | 浓硝酸 |
A. ①③ B. ①④ C. ②④ D. ②③