��Ŀ����
A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�ء���֪Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��Bλ��Ԫ�����ڱ���s����CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn��1npn��1�� Dԭ��M�ܲ�Ϊȫ����״̬����������ɶԵ��ӣ�EΪ��������δ�ɶԵ���������Ԫ�ء���ش��������⣺
��1��д��E��̬ԭ�ӵĵ����Ų�ʽ ��E�����ڱ��е�λ���� �����̬ԭ���� ��������ͬ�ĵ��ӡ�
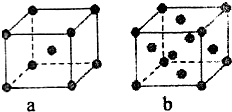
��2��ijͬѧ����������Ϣ���ƶ�B�ĺ�������Ų�����ͼ��ʾ��
��ͬѧ�����ĵ����Ų�ͼΥ���� ��
��3����֪A��C�γɵĻ�����X��ÿ��ԭ�ӵ�������Ϊ8�����ȶ��ṹ����X�Ļ�ѧʽΪ ��X��һ�ֳ�Ӳ���ʣ���ĥ�𡢿���ʴ����ǿ���Ʋ�X�ľ�������Ϊ ��
��4��B�ĵ�����A����ۺ������ϡ��Һ��Ӧ���ܽ�A��ԭ����ͼ�̬��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��5�� ��֪D��������Ӷѻ���ʽΪ�����������ܶѻ������þ�����һ�������ı߳�Ϊa cm����D������ܶ�Ϊ g/cm3��д����a�ı���ʽ����NA��ʾ�����ӵ�������ֵ������D��ԭ�Ӱ뾶Ϊr ������D��������r��ʾ�����ֶѻ�ģ�͵Ŀռ�������Ϊ ������ʽ��ʾ�����軯��
��1��1s22s22p63s23p63d54s1 ��4���ڵڢ�B�� 7
��2�� �������ԭ�� ��3��Si3N4 ԭ�Ӿ���
��4��4Mg + 10HNO3�� 4Mg(NO3)2 + NH4NO3 + 3H2O
��5��256/a3NA 4�� r3/ (2
r3/ (2 r)3
r)3
�������������Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯���������˵��AӦ���ǵ�Ԫ�ء�B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��Bλ��Ԫ�����ڱ���s��������B��þԪ�ء�CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn��1npn��1�������ܣ�2����n��3������C�ǹ�Ԫ�ء�Dԭ��M�ܲ�Ϊȫ����״̬����������ɶԵ��ӣ���˵��D��ͭԪ�ء�EΪ��������δ�ɶԵ���������Ԫ�أ�����E�Ǹ�6Ԫ�ء�
��1�����ݹ���ԭ����֪����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽ1s22s22p63s23p63d54s1 ������ԭ��������24��λ�����ڱ��ĵ�4���ڵڢ�B�塣����ͬһ�ܲ��в�ͬ�ܼ�������Ҳ�Dz���ͬ�ģ����Ը�Ҳ��Ԫ�ػ�̬ԭ����7��������ͬ�ĵ��ӡ�
��2�����ݹ������ʽ��֪��ͬѧ�����ĵ����Ų�ͼΥ�����������ԭ����
��3��A��C�γɵĻ�����X��ÿ��ԭ�ӵ�������Ϊ8�����ȶ��ṹ������Siλ�ڵڢ�A�壬��Ԫ��λ�ڵڢ�A�壬��X�Ļ�ѧʽSi3N4������X���������ʿ�֪�γɵľ�����ԭ�Ӿ��塣
��4����Ԫ�ص���ͼ��ǣ�3�ۣ������仹ԭ����������泥���Ӧ�Ļ�ѧ����ʽ��4Mg + 10HNO3�� 4Mg(NO3)2 + NH4NO3 + 3H2O��
��4���������嶥�����Ϊ8���������У������ĵ�Ϊ2���������У�������Ϊ��8��1/8 + 6��1/2 = 4���þ�����һ�������ı߳�Ϊa cm���� ������ܶȣ�256/a3NA��
������ܶȣ�256/a3NA��
���ݾ����ṹ��֪��4r�� �����a��
�����a��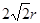
������������Ϊ
������4������ԭ�ӵ����Ϊ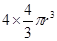
���Դ˾�����ԭ�ӿռ�ռ������4�� r3/(2
r3/(2 r)3
r)3
���㣺�����������Ų����������ʽ���������͡�����ṹ���жϺͼ���
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����Ӧ�������������������������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������


 CH3COOH+OH-
CH3COOH+OH-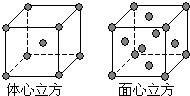 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㣮C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㣮C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ�� ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��
ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��
 ��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ������Ԫ�أ�����ԭ�������Ĵ�С��ϵΪA��C��B��D��E����֪Aԭ�ӵ�p���Ϊ����������γɵ��⻯��ķе���ͬ����ǽ���Ԫ�ص��⻯������ߵģ�Dԭ�ӵõ�һ�����Ӻ���3p�����ȫ������B+���ӱ�Dԭ���γɵ�������һ�����Ӳ㣮C��B���γ�BC�͵����ӻ����E��ԭ������Ϊ29��
��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ������Ԫ�أ�����ԭ�������Ĵ�С��ϵΪA��C��B��D��E����֪Aԭ�ӵ�p���Ϊ����������γɵ��⻯��ķе���ͬ����ǽ���Ԫ�ص��⻯������ߵģ�Dԭ�ӵõ�һ�����Ӻ���3p�����ȫ������B+���ӱ�Dԭ���γɵ�������һ�����Ӳ㣮C��B���γ�BC�͵����ӻ����E��ԭ������Ϊ29��