��Ŀ����
����0.1mol?L-1��Na2SO4��0.2moL?L-1��H2SO4�����Һl00mL����������μ���0.2moL?L-1��Ba��OH��2��Һ�������Ͻ��裬ʹ��Ӧ��ֽ��У�
��1��������100mLBa��OH��2��Һʱ��������Һ�е�������______��д��ѧʽ����������Ϊ______
��2������Һ�г������ﵽ���ʱ������Ba��OH��2��Һ�����Ϊ______mL��������Һ������Ϊ______��д��ѧʽ��������������ʵ���Ũ��Ϊ______mol?L-1 �����һ������Ҫд��������̣���Һ��Ϻ�������ԼӺͣ�
�⣺��1����Ӧ�൱��Ba��OH��2����H2SO4��Ӧ��Ȼ����Na2SO4�뷴Ӧ��100mLBa��OH��2��Һ��n[Ba��OH��2]=0.1L��0.2moL?L-1=0.02mol��100mL��Һ��n��H2SO4��=0.1L��0.2moL?L-1=0.02mol������������������ǡ�÷�Ӧ�������Ʋ���Ӧ����Һ������ΪNa2SO4��Na2SO4�����ʵ���Ϊ0.1L��0.1moL?L-1=0.01mol��Na2SO4������Ϊ0.01mol��142g/moL=1.42g��
�ʴ�Ϊ��Na2SO4��1.42g��
��2����Һ�г������ﵽ���ʱ���������ȫ��Ӧ������Ba2++SO42-=BaSO4������n[Ba��OH��2]=n��SO42-��=0.02mol+0.01mol=0.03mol��������������Һ�����Ϊ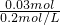 =0.15L=150mL��
=0.15L=150mL��
��ʱ��Һ������ΪNaOH�������������غ��֪n��NaOH��=n��Na+��=0.01mol��2=0.02mol������Һ��NaOHŨ��Ϊ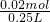 =0.08mol/L��
=0.08mol/L��
�ʴ�Ϊ��150��NaOH��0.08��
��������1����Ӧ�൱��Ba��OH��2����H2SO4��Ӧ��Ȼ����Na2SO4�뷴Ӧ��100mLBa��OH��2��Һ��n[Ba��OH��2]=0.1L��0.2moL?L-1=0.02mol��100mL��Һ��n��H2SO4��=0.1L��0.2moL?L-1=0.02mol������������������ǡ�÷�Ӧ�������Ʋ���Ӧ������n=cV���������Ƶ����ʵ������ٸ���m=nM���������Ƶ�������
��2����Һ�г������ﵽ���ʱ������Ba2++SO42-=BaSO4������n[Ba��OH��2]=n��SO42-�����ٸ���V=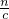 ���������������������Һ������ΪNaOH�������������غ��֪n��NaOH��=n��Na+�����ٸ���c=
���������������������Һ������ΪNaOH�������������غ��֪n��NaOH��=n��Na+�����ٸ���c= ���㣮
���㣮
���������⿼��������йؼ��㡢���û�ѧ�������йؼ���ȣ��ѶȲ������ⷢ����Ӧ�ı����ǽ���Ĺؼ���
�ʴ�Ϊ��Na2SO4��1.42g��
��2����Һ�г������ﵽ���ʱ���������ȫ��Ӧ������Ba2++SO42-=BaSO4������n[Ba��OH��2]=n��SO42-��=0.02mol+0.01mol=0.03mol��������������Һ�����Ϊ
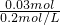 =0.15L=150mL��
=0.15L=150mL����ʱ��Һ������ΪNaOH�������������غ��֪n��NaOH��=n��Na+��=0.01mol��2=0.02mol������Һ��NaOHŨ��Ϊ
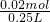 =0.08mol/L��
=0.08mol/L���ʴ�Ϊ��150��NaOH��0.08��
��������1����Ӧ�൱��Ba��OH��2����H2SO4��Ӧ��Ȼ����Na2SO4�뷴Ӧ��100mLBa��OH��2��Һ��n[Ba��OH��2]=0.1L��0.2moL?L-1=0.02mol��100mL��Һ��n��H2SO4��=0.1L��0.2moL?L-1=0.02mol������������������ǡ�÷�Ӧ�������Ʋ���Ӧ������n=cV���������Ƶ����ʵ������ٸ���m=nM���������Ƶ�������
��2����Һ�г������ﵽ���ʱ������Ba2++SO42-=BaSO4������n[Ba��OH��2]=n��SO42-�����ٸ���V=
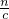 ���������������������Һ������ΪNaOH�������������غ��֪n��NaOH��=n��Na+�����ٸ���c=
���������������������Һ������ΪNaOH�������������غ��֪n��NaOH��=n��Na+�����ٸ���c= ���㣮
���㣮���������⿼��������йؼ��㡢���û�ѧ�������йؼ���ȣ��ѶȲ������ⷢ����Ӧ�ı����ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
�����Ŀ
��һ������0.1mol?L-1�Ĵ�����Һ������pH��ֽ�ⶨ��Һ��pH������ȷ�IJ����� ��������Һ�ʼ��Ե�ԭ���ǣ������ӷ���ʽ��ʾ�� ��
����������Dz�ͬ�¶���ˮ�����ӻ����ݣ�
�Իش����¼������⣺
��1����25��t1��t2����a 1��10-14�����������������=�������ɴ��жϵ������ǣ� ��
��2����t1���£�pH=10��NaOH��Һ�У�ˮ���������[OH-]Ϊ�� ��
��3����t2���£���pH=11�Ŀ�������ҺV1L��pH=1��������ҺV2L��ϣ����Ϻ���Һ���Ϊԭ����Һ���֮�ͣ�������Һ��pH=2����V1��V2= ������Һ�и������ӵ�Ũ���ɴ�С��˳���� ��
����������Dz�ͬ�¶���ˮ�����ӻ����ݣ�
| �¶�/�� | 25 | t1 | t2 |
| Kw/mol2?L-2 | 1��10-14 | a | 1��10-12 |
��1����25��t1��t2����a
��2����t1���£�pH=10��NaOH��Һ�У�ˮ���������[OH-]Ϊ��
��3����t2���£���pH=11�Ŀ�������ҺV1L��pH=1��������ҺV2L��ϣ����Ϻ���Һ���Ϊԭ����Һ���֮�ͣ�������Һ��pH=2����V1��V2=
