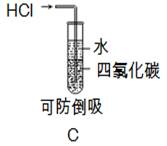
��Ŀ����
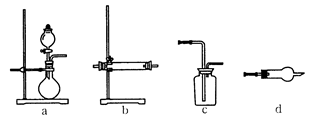
ijͬѧΪ��̽�����������ķ�Ӧ�����װ�����ͼ��ʾ��װ�ã����������ѹǿ����ʱ��ע��ˮ��Һ��Ŀ̶ȣ��ú��ᣩ��ȡ����Ƥ�����ڲ���ȼ�ճ��м�����ۣ��þƾ��Ƶ�ȼѸ��������ƿ�в�������Ƥ������۰�����ȼ�գ�ˮ����������ܡ�������Ϩ����ã�ˮ�����������ػص�ԭ�ȱ궨�Ŀ̶ȡ���Ҫ�ش��������⣺
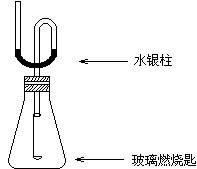
��1��ˮ�����������˵��ʲô��
_______________________________________________��
��2�����δȼ��ʱ�����Ϩ���ˣ�˵��_________________________________��
��3������ˮ��������ֻص�ԭ�ȱ궨�Ŀ̶ȣ��ɵõ�ʲô���ۣ�
__________________________________________________________________��
��4�����ݷ�Ӧ����ʽS + O2 ="=" SO2�����������ֿ��Ƶ���ʲô������֤��ʲô����
_______________________________________________________________________��

��1��ˮ�����������˵��ʲô��
_______________________________________________��
��2�����δȼ��ʱ�����Ϩ���ˣ�˵��_________________________________��
��3������ˮ��������ֻص�ԭ�ȱ궨�Ŀ̶ȣ��ɵõ�ʲô���ۣ�
__________________________________________________________________��
��4�����ݷ�Ӧ����ʽS + O2 ="=" SO2�����������ֿ��Ƶ���ʲô������֤��ʲô����
_______________________________________________________________________��
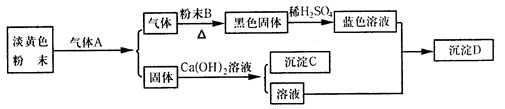
��1���ٸ÷�Ӧ�Ƿ��ȷ�Ӧ����������������
��2��ƿ�������Ѻľ�
��3����ͬ��ͬѹ�£���Ӧ���ĵ����������ɵ�SO2�����ͬ����ѹǿ��ͬ�������ʵ�����ͬ
��4��ͬ��ͬѹ�£���ͬ��������庬����ͬ�ķ�����
��2��ƿ�������Ѻľ�
��3����ͬ��ͬѹ�£���Ӧ���ĵ����������ɵ�SO2�����ͬ����ѹǿ��ͬ�������ʵ�����ͬ
��4��ͬ��ͬѹ�£���ͬ��������庬����ͬ�ķ�����
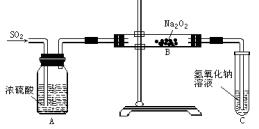
������ϵ����ѧ������ѹǿ֪ʶ�����������ѧ��Ӧ��ˮ�����Ĵ��ڣ�����ƿ�������Ϊһ�ܱ���ϵ����ˮ�����������ʱ��˵����ƿ���������ͣ����ڷ�Ӧ��S + O2 ="=" SO2��������Ӧǰ���������û�б仯�����Ψһ�Ŀ��ܾ��Ǹ÷�Ӧ���ȡ�
����һ��ʱ�����ƿ������ͨ�������������������ȴ��ݣ��ָ���ԭ���¶ȣ���ʱ����������ԭ�������һ������ѹǿû�иı䣬���ˮ�����ֻص�ԭ�ȱ궨�Ŀ̶ȡ�
����һ��ʱ�����ƿ������ͨ�������������������ȴ��ݣ��ָ���ԭ���¶ȣ���ʱ����������ԭ�������һ������ѹǿû�иı䣬���ˮ�����ֻص�ԭ�ȱ궨�Ŀ̶ȡ�

��ϰ��ϵ�д�
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
�����Ŀ

 �ⶨʣ���������
�ⶨʣ���������