��Ŀ����
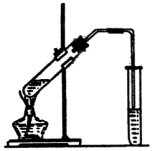
��֪�������ݣ�
ʵ������ȡ������������Ҫ�������£�
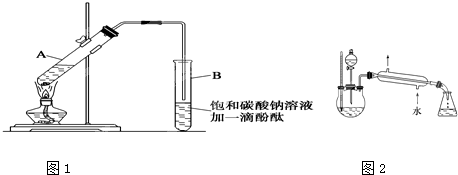
1.��30mL�Ĵ��Թ�A
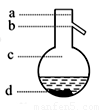
 �а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��
�а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ����Һ��2.��ͼ1���Ӻ�װ�ã�װ�����������ã�����С����ȵؼ���װ�л����Һ�Ĵ��Թ�5~10min��
3.���Թ�B�ռ���һ���������ֹͣ���ȣ������Թ�B��������Ȼ���ô��ֲ㡣
4.��������������㣬ϴ�ӡ����
�������ĿҪ��ش��������⣺
��1�����Ƹû����Һ����Ҫ��������Ϊ��_____��д����ȡ���������Ļ�ѧ����ʽ��______________��
��2������2������С����ȼ��ȣ�����Ҫ�����ǣ�__________________________��
��3��ָ������3�����۲쵽������_________������������������һ���ñ���ʳ��ˮ�ͱ����Ȼ�����Һϴ�ӣ���ͨ��ϴ�ӳ�ȥ�������ƣ�_________���ʣ�Ϊ�˸�������������ѡ�õĸ����Ϊ������ĸ����__________��
A.P2O5 B.��ˮNa2SO4
C.��ʯ�� D.����NaOH
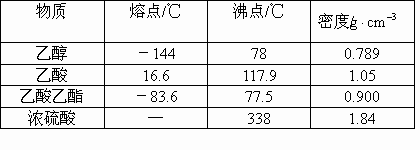
��4��ij��ѧ����С���������ͼ2��ʾ����ȡ����������װ�ã�ͼ�е�����̨�����С�����װ������ȥ������ͼ1װ����ȣ�ͼ2װ�õ���Ҫ�ŵ��У�_______________________��

������
�����������
��1�������Ҵ���Ũ���ᡢ������Һʱ�����Լ������Թܵ�˳������Ϊ��CH3CH2OH��ŨH2SO4��CH3COOH����ŨH2SO4�����Ҵ��У��ӱ�����Ϊ�˷�ֹ���ʱ�������������·ɽ��¹ʣ����Ҵ���ŨH2SO4�Ļ��Һ��ȴ�����������ϣ���Ϊ�˷�ֹ����Ļӷ����ԭ�ϵ���ʧ���ڼ���ʱ�Թ�����ʢ��Һ������ܳ����Թ��ݻ���1/3�����ݻ�Ϊ30mL����ô��ʢ��ҺΪ10mL���������1��4��4�ı�������Ũ���ᡢ������Ҵ��Ļ����Һ���ɴ˿�֪����Ӧ��Ũ���ᡢ������Ҵ�������ֱ�ΪlmL��4mL��4mL��
��2�������и����ĸ����ʵ���Ҫ���������ʿ�֪�����ᣨ117.9�棩���Ҵ���78.0�棩�ķе㶼�Ƚϵͣ��������������ķе㣨75.5�棩�ȽϽӽ������ô����ȣ���Ӧ�����������������������һ��������������ԭ�ϵĴ�����ʧ����һ���棬�¶�̫�ߵĻ������ܻᷢ����������Ӧ��
��3���ڲ���3�е���Ҫ�����ǣ��Թ�B�е�Һ��ֳ��������㣬�ϲ���״Һ��ɫ�������ŵ�ˮ����ζ�����²�Һ�壨dz����ɫ�����²�Һ��ĺ�ɫ��dz������������Ǵֲ�Ʒ���������㣬���������ֲ�Ʒ���ᴿ�����ᴿ����Ϊ������ֲ�Ʒ�м���̼���Ʒ�ĩ��Ŀ���dz�ȥ�ֲ�Ʒ�е����ᣩ���������м��뱥��ʳ��ˮ�뱥���Ȼ�����Һ�������á���Һ��Ŀ���dz�ȥ�ֲ�Ʒ�е��Ҵ���δ��Ӧ���̼�������ʣ����������м�����ˮ�����ƣ�Ŀ���dz�ȥ�ֲ�Ʒ�е�ˮ����������������������Һ�������һ�����������ƿ�ڣ�����������ȥ�ͷе���֣��ռ��¶���76��78��֮�����ּ��ô�������������
��4���Ա�����ʵ��װ��ͼ��������������Ʊ������еĸ����������ƣ����Կ������ߵ�����ͻ�����ŵ㣺���������¶ȼƣ����ڿ��Ʒ���װ���з�ӦҺ���¶ȣ����ٸ�����IJ������������˷�Һ©���������ڼ�ʱ���䷴Ӧ���Һ����������������IJ�������������ˮ����װ�ã��������ռ���������������

��ϰ��ϵ�д�
 �ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д�
�ǻۿ����ܾ�100�ֵ�Ԫ���ؼ��ϵ�д� ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�
�����Ŀ