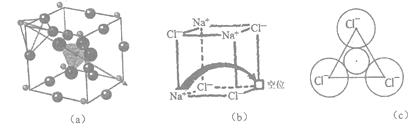
��Ŀ����
����Ŀ����֪�л���A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ�����ľۺϷ�Ӧ��Ʒ���ִ��ճ���������;�ܹ㡣һ�������£�A��ˮ��Ӧ����B��B�׳ƾƾ���B��ͭ�����ȴ��������¿��Ա���������ΪC�������������Ը��������Һ��Ӧ��ֱ������ΪD��B��Dһ�������·�Ӧ�IJ���E���������ϡ��ǹ�����ˮ�ͻ�ױƷ�е����ϡ�
�ش��������⣺
��1��A�����й����ŵ�������___��A�����������ӳɺ����F�ķ���ʽΪ___����F��Ϊͬϵ���������̼ԭ����Ϊ4���л�����һ�ȴ�����___�֡�
��2��д��B��D��Ӧ����E�Ļ�ѧ����ʽΪ___���÷�Ӧ�ķ�Ӧ����Ϊ___��
��3��B������ȼ�ϵ�أ�����NaOH���������Һ�������缫��Ӧ����ʽΪ___��
���𰸡�̼̼˫�� C2H6 4 CH3COOH+C2H5OH![]() CH3COOC2H5+H2O ������Ӧ����ȡ����Ӧ�� CH3CH2OH-12e-+16OH-=2CO
CH3COOC2H5+H2O ������Ӧ����ȡ����Ӧ�� CH3CH2OH-12e-+16OH-=2CO![]() +11H2O
+11H2O
��������
�л���A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪ��ϩ��һ�������£�A��ˮ��Ӧ����B��B�׳ƾƾ����Ҵ���B��ͭ�����ȴ��������¿��Ա���������ΪC��CΪ��ȩ�������Ը��������Һ��Ӧ��ֱ������ΪD��DΪ���ᣬB��Dһ�������·�Ӧ�IJ���EΪ����������
(1)A����Ϊ��ϩ�������ŵ�������̼̼˫������ϩ�����������ӳɺ����F�ķ���ʽΪC2H6�������黥Ϊͬϵ���������̼ԭ����Ϊ4���л���Ϊ��������춡�飬�����л���ĺ˴Ź���������2�֣����Զ����һ�ȴ�����4�֡�
(2)B��D��Ӧ����E�Ļ�ѧ����ʽΪCH3COOH+C2H5OH![]() CH3COOC2H5+H2O���÷�Ӧ�ķ�Ӧ����Ϊ������Ӧ����ȡ����Ӧ����
CH3COOC2H5+H2O���÷�Ӧ�ķ�Ӧ����Ϊ������Ӧ����ȡ����Ӧ����
(3)B������ȼ�ϵ�أ�����NaOH���������Һ����������������Ӧ�����������̼���������Ʒ�Ӧ����̼������ӣ��缫��Ӧ����ʽΪCH3CH2OH-12e-+16OH-=2CO![]() +11H2O��
+11H2O��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
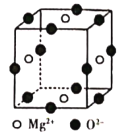
Сѧ��10����Ӧ����ϵ�д�����Ŀ����ˮ�к��зḻ��þ��Դ��ijͬѧ����˴�ģ�⺣ˮ���Ʊ�MgO��ʵ�鷽����

ģ�⺣ˮ�е�����Ũ��/mol��L��1 | Na�� | Mg2�� | Ca2�� | Cl�� | HCO3�� |
0.439 | 0.050 | 0.011 | 0.560 | 0.001 |
ע����Һ��ij�����ӵ�Ũ��С��1.0��10��5 mol��L��1������Ϊ�����Ӳ����ڣ�ʵ������У�������Һ������䡣Ksp[CaCO3]��4.96��10��9��Ksp[MgCO3]��6.82��10��6��Ksp[Ca(OH)2]��4.68��10��6��Ksp[Mg(OH)2]��5.61��10��12������˵����ȷ���ǣ� ��
A.������XΪCaCO3
B.��ҺM�д���Mg2����������Ca2��
C.��ҺN�д���Mg2����Ca2��
D.�����������Ϊ����4.2 g NaOH���壬������YΪCa(OH)2��Mg(OH)2�Ļ����
����Ŀ�������£���һԪ��HA����Һ��KOH��Һ�������ϣ���������仯����ʵ���������±���
ʵ���� | ��ʼŨ��/��mol��L��1�� | ��Ӧ����Һ��pH | |
c��HA�� | c(KOH) | ||
�� | 0.1 | 0.1 | 9 |
�� | x | 0.2 | 7 |
�����жϲ���ȷ����
A��ʵ������Ӧ�����Һ�У�c(K��)>c(A��)>c(OH��)>c(H��)
B��ʵ������Ӧ�����Һ�У�c(OH��)=c(K��)��c(A��)=![]() mol/L
mol/L
C��ʵ������Ӧ�����Һ�У�c(A��)��c(HA)>0.1mol��L��1
D��ʵ������Ӧ�����Һ�У�c(K��)=c(A��)>c(OH��) =c(H��)