��Ŀ����
����Ŀ�����ڶ������Դ�У����������Ϊ21�������������Դ����ͼ��ʾ�����Ṥҵ�в�����S02ͨ�����й��̼����Ƶ�H2O,�����Ƶ�H2��

��ش�
(1)�ù�����X ��_____________(д��ѧʽ����
(2)д���ù��̷�Ӧ�Ļ�ѧ����ʽ��I _________ ,II ______________ ��
(3)β���е�S02����NaOH��Һ���գ�ͬʱ�ɵú�Na2S03����Ʒ��Ϊ�ⶨ��Ʒ�� Na2SO3��������������ͬѧ���ʵ������(�гּ�����װ����):

��Aװ�÷�����Ӧ�Ļ�ѧ����ʽ __________��
��װ��D�������� _______________ ��
�۳���ͨ�뵪����Ŀ��_______________ ��
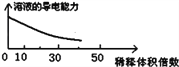
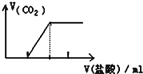
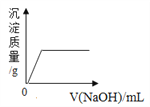
������Ʒ�е�����������������ݳ���Na2SO3����Ʒ�����⣬����Ҫ�� _______��
���𰸡� I2 SO2+I2+2H2O=H2SO4+2HI 2HIH2+I2 Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O ��ֹ�����е�H2O��CO2��O2����C�� �ž�װ���еĿ�����ͬʱʹSO2 ��Cװ������Һ������� װ��Cͨ��SO2 ǰ�������
������������������̺���Ϣ֪����Ӧ��ΪSO2��������SO2+I2+2H2O��H2SO4+2HI����Ӧ��Ϊ��2HIH2 + I2��
��1���������Ϸ�����֪X��I2����2���������Ϸ�����֪��Ӧ��Ϊ��SO2+I2+2H2O��H2SO4+2HI����Ӧ��Ϊ��2HIH2+ I2����3����ʵ��ķ�Ӧԭ��Ϊ��Na2SO3+H2SO4��Ũ����Na2SO4+SO2��+H2O��2NaOH+SO2��Na2SO3+H2O��ͨ���ⶨװ��C��Ӧǰ��������������������������Ƶ���������
�ٸ������Ϸ�����֪Aװ�÷�����Ӧ�Ļ�ѧ����ʽΪNa2SO3+H2SO4��Ũ����Na2SO4+SO2��+H2O�������ڿ����е�ˮ������CO2���������ᱻCװ�����գ�����װ��D�������Ƿ�ֹ�����е�H2O��CO2��O2����C����������װ���к��п�����ͬʱ�����SO2�����Գ���ͨ�뵪����Ŀ�����ž�װ���еĿ�����ͬʱʹSO2 ��Cװ������Һ����������ܸ������Ϸ�����֪�ⶨ��Ʒ��Na2SO3��������������������Ǻ�Na2SO3����Ʒ������װ��Cͨ��SO2 ǰ���������

����Ŀ���±��г��˳�ȥ�������������ʵķ��������д�����ǣ� ��
ѡ�� | ���� | �������� | ��ȥ���ʵķ��� |
A | CO | CO2 | ͨ��������NaOH��Һ������ |
B | NaCl | ��ɳ | �ܽ⡢���ˡ������ᾧ |
C | NaOH��Һ | Na2CO3 | ����ϡ���������ٲ������� |
D | Cu(NO3)2 | AgNO3 | ���������ͭ�ۣ����� |
A. A B. B C. C D. D