��Ŀ����
��14�֣���ͨ������������п��ˮ�����õ�ȩ��ͪ��
���磺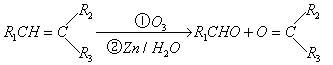
������Ӧ�������ƶ�ϩ���Ľṹ��һ����״��ϩ��Aͨ������������п��ˮ�����õ�B��C��������B����Է���������58����̼62.1%������10.3%��B��������Ӧ������������D��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ������E����Ӧͼʾ���£�
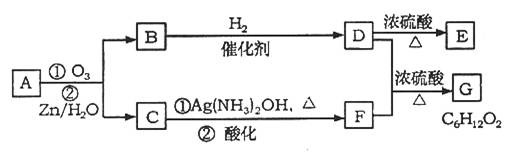
�ش��������⣺
��1��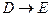 �ķ�Ӧ����Ϊ_____________��C�к��й����ŵ�����Ϊ___________��B�Ľṹ��ʽΪ _________________��
�ķ�Ӧ����Ϊ_____________��C�к��й����ŵ�����Ϊ___________��B�Ľṹ��ʽΪ _________________��
��2��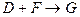 �Ļ�ѧ����ʽ��__________________________________��
�Ļ�ѧ����ʽ��__________________________________��
��3��A�Ľṹ��ʽΪ__________________________________________��
��4��������A��ij��ͬ���칹��ͨ������������п��ˮ����ֻ�õ�һ�ֲ��д�����з��ϸ�������ͬ���칹��Ľṹ��ʽ__________________��_____________��
���磺
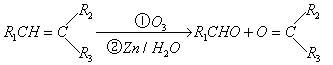
������Ӧ�������ƶ�ϩ���Ľṹ��һ����״��ϩ��Aͨ������������п��ˮ�����õ�B��C��������B����Է���������58����̼62.1%������10.3%��B��������Ӧ������������D��D��Ũ��������¼��ȣ��ɵõ���ʹ��ˮ��ɫ������E����Ӧͼʾ���£�

�ش��������⣺
��1��
 �ķ�Ӧ����Ϊ_____________��C�к��й����ŵ�����Ϊ___________��B�Ľṹ��ʽΪ _________________��
�ķ�Ӧ����Ϊ_____________��C�к��й����ŵ�����Ϊ___________��B�Ľṹ��ʽΪ _________________����2��
 �Ļ�ѧ����ʽ��__________________________________��
�Ļ�ѧ����ʽ��__________________________________����3��A�Ľṹ��ʽΪ__________________________________________��
��4��������A��ij��ͬ���칹��ͨ������������п��ˮ����ֻ�õ�һ�ֲ��д�����з��ϸ�������ͬ���칹��Ľṹ��ʽ__________________��_____________��
��1����ȥ��Ӧ��ȩ����CH3COCH3��ÿ��2�֣�
��2��CH3CH2COOH+ CH3CH (OH)CH3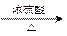 CH3CH2COOCH(CH3)2��H2O��2�֣�
CH3CH2COOCH(CH3)2��H2O��2�֣�
��3�� CH3CH2CH=C(CH3)2 ��2�֣�
��4��CH3CH2CH=CHCH2CH3 ��2�֣� (CH3)2C=C(CH3)2��2�֣�
��2��CH3CH2COOH+ CH3CH (OH)CH3
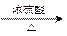 CH3CH2COOCH(CH3)2��H2O��2�֣�
CH3CH2COOCH(CH3)2��H2O��2�֣���3�� CH3CH2CH=C(CH3)2 ��2�֣�
��4��CH3CH2CH=CHCH2CH3 ��2�֣� (CH3)2C=C(CH3)2��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ



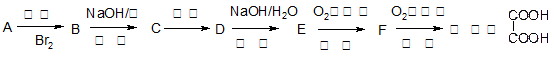
 �ش��������⣺
�ش��������⣺ ��
�� ��
��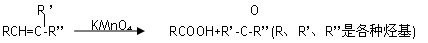
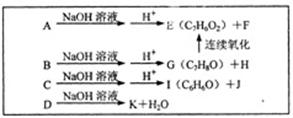
 ��˵���У���ȷ����
��˵���У���ȷ����
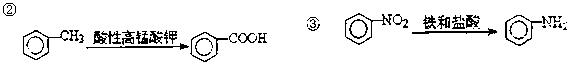

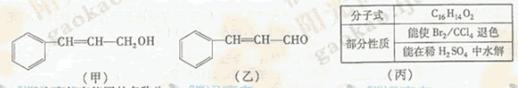
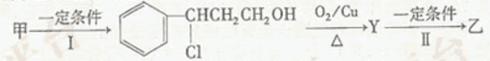
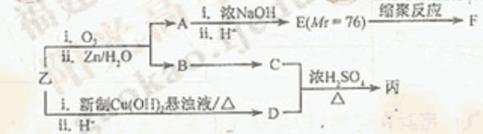

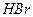 c��
c�� 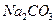 ��Һ d�� ����
��Һ d�� ����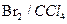 �����ӳɷ�Ӧ d����
�����ӳɷ�Ӧ d���� ��Һ��ʾ������ɫ
��Һ��ʾ������ɫ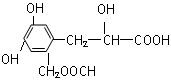 �������ܷ����ķ�Ӧ�У��ټӳɷ�Ӧ��
�������ܷ����ķ�Ӧ�У��ټӳɷ�Ӧ��