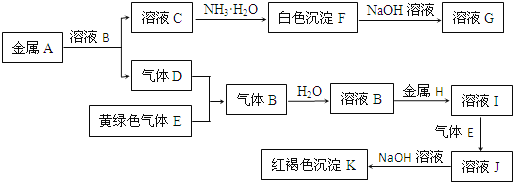
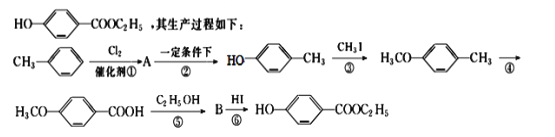
��Ŀ����
����Ŀ�������A��B��C���������й���Ϣ��
A | B | C |
����ʹ������� ��̼��Һ��ɫ�� �ڱ���ģ��Ϊ��
| ��ƽ���ͽṹ �ڹ�ģ��Ϊ�� | ����ʹ������Ȼ�̼��Һ��ɫ�� ��1mol����2molH2��һ�������·�Ӧ ����A �۱���ģ��Ϊ�� |
ͼ1 |
|
���ݱ�����Ϣ�ش��������⣺
��1����ͼ1����B��Һ����ȡ�屽��װ�ã��Իش�
��a��װ��C�е�����___________________________________��
��b��װ��B������__________________________________��
��2����ͼ2����ȡ��C��װ�ã��Իش�
��a��Ϊ�˿���������Ȳ�����ʣ���Һ©����ʢ�ŵ�Һ����_______________��
��b������Һ�����ƿ�з�����Ӧ������Ȳ��д���˷�Ӧ�Ļ�ѧ����ʽ��_________��
��c����ʯ�к��������ʣ�����H2S,PH3�����壬Ϊ�˲�����Ȳ������ɸ��ţ�Ӧ�ó��ӣ�һ��ѡ��___________ϴ����
���𰸡� ���ܿ��а������֣���Һ���ֵ���ɫ������ ���ջӷ��������壮 ����ʳ��ˮ CaC2+2H2O��Ca��OH��2+CH��CH���� CuSO4����NaOH��Һ
���������ɱ���ģ�Ϳ�֪AΪ��ϩ��BΪ����CΪ��Ȳ��
(1)(a)��������������Ӧ����dz��ɫ���廯�������ᣬ���廯�����������C�������˵���ɫ������֤����Ӧ���������廯�⣬�ҵ��ܿ��а������֣�
(b)���ӷ������������Ȼ�̼�������Ȼ�̼���ջӷ��������壻��
(2)(a)ʹ�ñ���ʳ��ˮ����ˮ���ɿ���������Ȳ�����ʣ����Һ©����ʢ�ŵ�Һ���DZ���ʳ��ˮ��
(b)ˮ��̼���Ʒ�Ӧ������Ȳ���������ƣ��˷�Ӧ�Ļ�ѧ����ʽΪCaC2+2H2O��Ca(OH)2+CH��CH����
(c)ѡ��CuSO4����NaOH��Һ�ɳ�ȥ��Ȳ�л��е�H2S��PH3�����壬���������Ȳ���ʵļ��顣