��Ŀ����
����Ŀ������ij��Ȼ��֬ A �ķ���ʽΪ C57H106O6��1mol����֬ˮ��ɵõ� 1mol ���͡�1mol ������֬���� B ��2mol ֱ������֬���� C�����ⶨ B����Է�������Ϊ 280��ԭ�Ӹ�����Ϊ C��H��O��9��16��1��
��1��д�� B�ķ���ʽ��______________________________��
��2��д�� C�Ľṹ��ʽ��____________________��C ��������____________________��
��3��д����5 ��̼ԭ�ӵ� C ��ͬϵ���ͬ���칹��Ľṹ��ʽ____________________��
����RCH��CHR������� KMnO4 ��Һ���Ⱥ��ữ������˫�������������
RCH��CHR��![]() RCOOH��R��COOH�������ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ���ڴ��������£�1mol ������֬���� B�� 1molH2��Ӧ�����õ� D �� E �Ļ���D �� E ��Ϊͬ���칹�塣�� D �� E �Ļ��������� KMnO4��Һ�����ữ�õ��������ֲ��
RCOOH��R��COOH�������ø÷�Ӧ�IJ��ﷴ�ƺ�̼̼˫��������Ľṹ���ڴ��������£�1mol ������֬���� B�� 1molH2��Ӧ�����õ� D �� E �Ļ���D �� E ��Ϊͬ���칹�塣�� D �� E �Ļ��������� KMnO4��Һ�����ữ�õ��������ֲ��
HCOOC��(CH2��10��COOHCH3��(CH2��7��COOH
HCOOC��(CH2��7��COOHCH3��(CH2��4��COOH
��4��д�� D�� E �Ľṹ��ʽ��______________________________��
��5��д�� B�Ľṹ��ʽ��______________________________��
��6��д����Ȼ��֬ A ��һ�ֿ��ܽṹ��ʽ��______________________________��
���𰸡�
��1��C18H32O2
��2��CH3-(CH2��16-COOH��Ӳ֬��(��ʮ�����ᡢʮ������
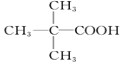
��3�� CH3CH2CH2CH2COOH��![]() ��
�� ��
��![]()
��4��CH3-(CH2��7-CH=CH-(CH2��7-COOH��CH3-(CH2��4-CH=CH-(CH2��10-COOH
��5��CH3-(CH2��4-CH=CH-CH2-CH=CH-(CH2��7-COOH
��6��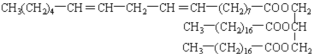 ��
��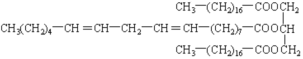
��������
�������������B����Է�������Ϊ280��ԭ�Ӹ�����ΪC��H��O=9��16��1����B�ķ���ʽΪC9nH16nOn��n=![]() =2������B�ķ���ʽΪ��C18H32O2��A��ˮ����Ա�ʾ�ɣ�C57H106O6+3H2O��C3H8O3(������+C18H32O2+2C������ԭ���غ�֪��C�ķ���ʽΪ��C18H36O2�����C��ֱ������֬���ᣬ��֪C�Ľṹ��ʽΪ��CH3-(CH2��16-COOH��
=2������B�ķ���ʽΪ��C18H32O2��A��ˮ����Ա�ʾ�ɣ�C57H106O6+3H2O��C3H8O3(������+C18H32O2+2C������ԭ���غ�֪��C�ķ���ʽΪ��C18H36O2�����C��ֱ������֬���ᣬ��֪C�Ľṹ��ʽΪ��CH3-(CH2��16-COOH��
��1��ͨ�����Ϸ���֪��B�ķ���ʽΪ��C18H32O2���ʴ�Ϊ��C18H32O2��
��2������ԭ���غ�֪��C�ķ���ʽΪ��C18H36O2���ʴ�Ϊ��C18H36O2��
��3����5��̼ԭ�ӵ�Ӳ֬���ͬϵ��Ϊ����(C4H9COOH����������ͬ���칹����4�֣����Ӧ������ҲӦ��4�֣���CH3CH2CH2CH2COOH��![]() ��
�� ��
��![]() ���ʴ�Ϊ��CH3CH2CH2CH2COOH��
���ʴ�Ϊ��CH3CH2CH2CH2COOH��![]() ��
�� ��
��![]() ��
��
����D��E��Ϊͬ���칹�壮��D��E������̼ԭ�Ӹ�����ȣ���D��E�Ļ���������KMnO4��Һ���Ⱥ��ữ���õ������ᣬ����D��E̼ԭ�Ӹ������֪��HOOC(CH2��10COOH��CH3(CH2��4COOH����ͬһ��ϩ���õ��ģ�CH3(CH2��7COOH��HOOC(CH2��7COOH����ͬһ��ϩ���õ��ģ������������Ȼ�ȥ��̼̼˫�����Ӽ��ɵõ���Ӧϩ����B�������������Ǽӳɷ�Ӧ��D��E��ͬ�Ļ���Ӧ�üӳɵĵط���ͬ�����ģ�����������Եõ�B������˫��CH3(CH2��4CH�TCHCH2CH�TCH(CH2��7COOH��
��4��ͨ�����Ϸ���֪��D��E�ṹ��ʽ�ֱ�ΪCH3(CH2��7CH�TCH(CH2��7COOH��CH3(CH2��4CH�TCH(CH2��10COOH���ʴ�Ϊ��CH3(CH2��7CH�TCH(CH2��7COOH��CH3(CH2��4CH�TCH(CH2��10COOH��
��5��ͨ�����Ϸ���֪��B�ṹ��ʽΪCH3(CH2��4CH�TCHCH2CH�TCH(CH2��7COOH���ʴ�Ϊ��CH3(CH2��4CH�TCHCH2CH�TCH(CH2��7COOH��
��6����Ȼ��֬ A���ܵĽṹ��ʽΪ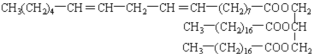 ��
��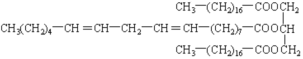 ���ʴ�Ϊ��
���ʴ�Ϊ��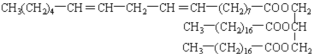 ��
��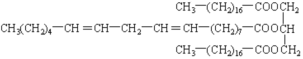 ��
��


