��Ŀ����
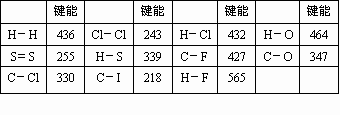
��˹������ָһ����ѧ��Ӧ������һ����ɻ��Ƿּ�����ɣ�________��һ���ģ�����ѧ��Ӧ���ʱ�ֻ�뷴Ӧ��________�йأ����뷴Ӧ��;���أ�
���ø�˹���ɼ��㻯ѧ��Ӧ�ʱ�ķ�������һ����ѧ����ʽ�������⼸����ѧ����ʽ��Ӽ����õ�������Щ��ѧ��Ӧ���ʱ���Ӧ________���������Ҫ����Ļ�ѧ��Ӧ���ʱ䣮
������
(1)��֪��C(s)��O2(g)��CO2(g)����H����393.5 kJ��mol��1��CO(g)��![]() O2(g)��CO2(g)����H����283.0 kJ��mol��1��д��C(s)ȼ������CO���Ȼ�ѧ����ʽ��___________��
O2(g)��CO2(g)����H����283.0 kJ��mol��1��д��C(s)ȼ������CO���Ȼ�ѧ����ʽ��___________��
(2)��֪��C(s)��![]() O2(g)��CO(g)����H����110.5 kJ��mol��1��2H2(g)��O2(g)��2H2O(g)����H����483.6 kJ��mol��1����ӦC(s)��H2O(g)��CO(g)��H2(g)�Ħ�HΪ
O2(g)��CO(g)����H����110.5 kJ��mol��1��2H2(g)��O2(g)��2H2O(g)����H����483.6 kJ��mol��1����ӦC(s)��H2O(g)��CO(g)��H2(g)�Ħ�HΪ
A��262.6 kJ��mol��1
B����131.3 kJ��mol��1
C����352.3 kJ��mol��1
D��131.3 kJ��mol��1
(3)��֪H2(g)��![]() O2(g)��H2O(l)����H����285.83 kJ��mol��1��CO(g)��
O2(g)��H2O(l)����H����285.83 kJ��mol��1��CO(g)��![]() O2(g)��CO2(g)����H����282.9 kJ��mol��1����������һ����̼�Ļ��������ȫȼ�տ�����5.4 gҺ̬ˮ�����ų�114.03 kJ������������������CO�����ʵ���Ϊ
O2(g)��CO2(g)����H����282.9 kJ��mol��1����������һ����̼�Ļ��������ȫȼ�տ�����5.4 gҺ̬ˮ�����ų�114.03 kJ������������������CO�����ʵ���Ϊ
A��0.22 mol����B��0.15 mol����C��0.1 mol����D��0.05 mol
(4)0.3 mol����̬������(B2H6)����ȼ����������ȼ�����ɹ�̬B2O3��Һ̬ˮ�����ų�649.5 kJ���������Ȼ�ѧ����ʽΪ________________________����֪H2O(l)��H2O(g)����H��44 kJ��mol��1����11.2 L��״������������ȫȼ��������̬ˮʱ�ų�������Ϊ________kJ��

| |||||||||||||||