��Ŀ����
�������糧�ͷų������ĵ�������(NOx)����������Ͷ�����̼���������ɻ�����Ⱦ����ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ġ�
(1)���������ü������ԭNOx��
CH4(g)��4NO2(g)===4NO(g)��CO2(g)��2H2O(g)����H1����574kJ��mol��1
CH4(g)��4NO(g)===2N2(g)��CO2(g)��2H2O(g)����H2����1160kJ��mol��1
����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ�� ����
(2)��̼����CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)��
CH3OH(g)��H2O(g)��
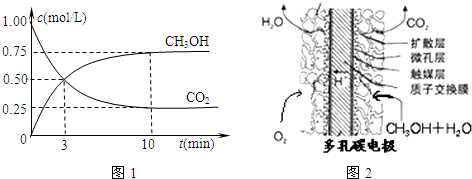
��ȡ��ݵ����CO2��H2�Ļ������(���ʵ���֮�Ⱦ�Ϊ1��3)���ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״������������(CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ��������ͼ��ʾ��������CO2ת��Ϊ�״��ķ�Ӧ�Ħ�H3
����0(�����������������)��

����һ���º����ܱ������г���1mol CO2��3mol H2������������Ӧ�����CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ��ʾ������˵����ȷ������(����ĸ����)��

A.��10min������������ٳ���1mol CO2��3mol H2�����ٴδﵽƽ��ʱc(CH3OH)��1.5mol��L��1
B.0��10min�ڣ�������ƽ����Ӧ����Ϊ0.075mol/(L��min)
C.�ﵽƽ��ʱ��������ת����Ϊ0.75
D.���¶��£���Ӧ��ƽ�ⳣ����ֵΪ3/16
E.�����¶Ƚ�ʹn(CH3OH)/n(CO2)��С
�ۼ״�ȼ�ϵ�ؽṹ����ͼ��ʾ���乤��ʱ�����ĵ缫��Ӧʽ�ɱ�ʾΪ��
��

(3)����ij���������н���������������һ�����İ�����������Ӧ����������狀�����淋Ļ������Ϊ����Ʒ���ʡ��������е�SO2��NO2�����ʵ���֮��Ϊ1��1����÷�Ӧ�Ļ�ѧ����ʽΪ ��
(4)����狀�����淋�ˮ��Һ��pH��7������ԭ�����һ�����ӷ���ʽ��ʾΪ����NH��H2O![]() NH3��H2O��H��������һ�����ʵ���Ũ�ȵ��������Һ�еμ�������NaOH��Һ��ʹ��Һ��pH��7������Һ�У�c(Na��)��c(H��)����c(NO)��c(OH��)(��д����������������)��
NH3��H2O��H��������һ�����ʵ���Ũ�ȵ��������Һ�еμ�������NaOH��Һ��ʹ��Һ��pH��7������Һ�У�c(Na��)��c(H��)����c(NO)��c(OH��)(��д����������������)��
(1)CH4(g)��2NO2(g)===N2(g)��CO2(g)��2H2O(g)����H����867kJ/mol��
(2) �٦�H3������0(�����������������)��
����CE��(����ĸ����)��
�ۡ�O2��4e����4H��===2H2O����
(3) 12NH3��3O2��4SO2��4NO2��6H2O===4(NH4)2SO4��4NH4NO3����
(4)������ (�����������������)��
����:
��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д� ��2011?��ƽ��ģ���������糧�ͷų������ĵ������NOx������������Ͷ�����̼���������ɻ�����Ⱦ����ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ģ�
��2011?��ƽ��ģ���������糧�ͷų������ĵ������NOx������������Ͷ�����̼���������ɻ�����Ⱦ����ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ģ�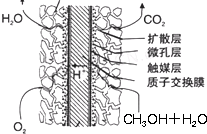
 NH3?H2O+H+
NH3?H2O+H+