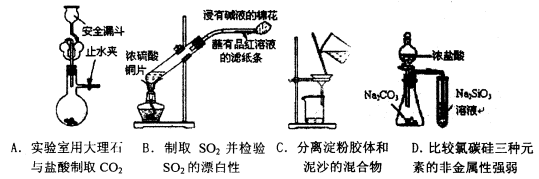
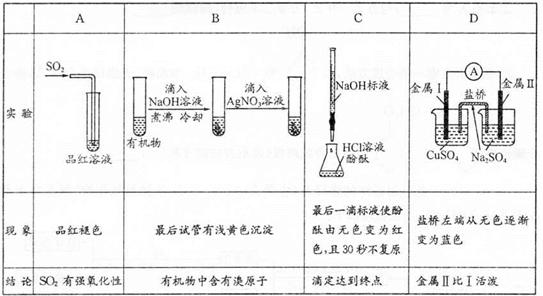
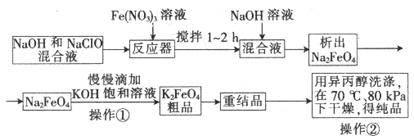
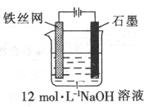
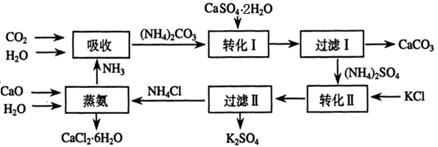
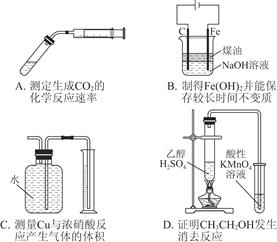
��Ŀ����
����ʵ�顰�����������롰���ۡ���Ӧ��ϵ��ȷ����
| | ���������� | ���� |
| A | ������¯ˮ���е�CaSO4ʱ�����μ��뱥��Na2CO3��Һ�����ᣬˮ���ܽ� | Ksp��CaCO3��CaSO4 |
| B | ��ʯī���缫���MgSO4��Һ��ij�缫�����а�ɫ�������� | �õ缫Ϊ���� |
| C | ��FeCl3��CuCl2�����Һ�м������ۣ��к�ɫ�������� | �����ԣ�Cu2+��Fe3+ |
| D | ��ij��Һ���ȵμ������ữ���ٵμ�BaCl2��Һ���а�ɫ�������� | ����Һ��һ������Ag+ |
A
�������������A������̼��Ƶ��ܶȻ�����С������Ƶ��ܶȻ�����������ݳ�������������ܵķ���ת����֪��ѡ��A��ȷ����ʯī���缫���MgSO4��Һ������������Һ�е�OH���ŵ磬��������ˮ�ĵ���ƽ�ⱻ�ƻ�����Һ�����ԡ���������Һ�е������ӷŵ磬��ͬ��������֪��������Χ��Һ�Լ��ԣ������������þ���ɣ�ѡ��B����ȷ����������Cu2+��Fe3+��ѡ��C����ȷ��ѡ��D�У���Һ��Ҳ���ܺ���SO42����SO32����D����ȷ����ѡA��
���㣺����ʵ�鷽��������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������߿����ۺ���ǿ������������ѧ���淶�Ͻ���ʵ����ơ�������������������ȷ���ʵ����ʼ������Ļ�ѧ��Ӧ�ǽ����Ĺؼ�������Ϥ������ԭ��Ӧ�����ӵļ���������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��˫ѡ������ʵ�鷽����ȷ����
| A������ˮ�Ҵ���ȡ��ˮ�еĵⵥ�� |
| B������ϡHNO3��BaCl2��Һ������ɫ��������Һ��һ����SO42�� |
| C������FeCl3��Һһ���Լ���CCl4��������Һ��NaI��Һ��NaCl��Һ |
| D��������������0.1mol/L NaCl��Һ������ᴿ��ʵ���������������ͬ |