��Ŀ����
ȫ����ÿ����������ʧ�ĸ���Լռ��������������1/4��ijѧ����̽��������������������ɾ��������ֱ�ͬʱ���A��B��C��֧�Թ��н����о���
��1�����������ѧ���������ʵ����Ƶ����ݣ�
��2��һ�ܺ��Ϊ________���Թ��е������������⡣֤��������������ǣ� ��
��3������ͬ����������������ͬʱ�������������ˣ���װ���½���³���Ȱ�װ��������غ������ ������ס����ѡ������⡣
��1�����������ѧ���������ʵ����Ƶ����ݣ�
| ��� | �������� | ʵ��Ŀ�� |
| A | | ̽�����ڸ�������е�������� |
| B | ����������ע������ˮ��û��������ֲ����Һ�� | |
| C | | ̽�������п�����ˮʱ��������� |
��3������ͬ����������������ͬʱ�������������ˣ���װ���½���³���Ȱ�װ��������غ������ ������ס����ѡ������⡣
��1��
��2��C ���ڳ�ʪ�Ļ����У����������Ӵ�
��3������
| ��� | �������� | ʵ��Ŀ�� |
| A | ���Ⱥ���Թܣ�С�ķ��������������ϼ�һЩ������������ | |
| B | | ̽��������ˮ��������������ʱ��������� |
| C | С�ķ���������ע������ˮ��ʹ�������ֽ���ˮ�� | |
��3������
��

��ϰ��ϵ�д�
�����Ŀ
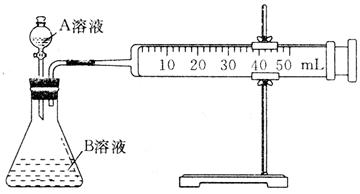
 �ʵĴ�С��ȡ��֧�Թܸ�����2
�ʵĴ�С��ȡ��֧�Թܸ�����2 0.1
0.1 H2C2O4��Һ����ȡ��֧�Թܸ�����4
H2C2O4��Һ����ȡ��֧�Թܸ�����4
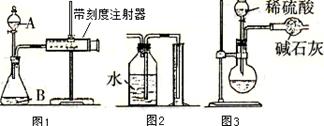
 ���˳����Բ��ò�ͬ��ұ�����������п����á��ȷֽⷨ��ұ���Ľ�����__________�� ��
���˳����Բ��ò�ͬ��ұ�����������п����á��ȷֽⷨ��ұ���Ľ�����__________�� ��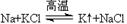 ���ƺ���Υ�����˽������˳���Խ������ܽ������õļ��û�������ԭ��__________________________ ��
���ƺ���Υ�����˽������˳���Խ������ܽ������õļ��û�������ԭ��__________________________ �� �����ݲ�������Һ��ɫ��dz��һ��ʱ�����Һ
�����ݲ�������Һ��ɫ��dz��һ��ʱ�����Һ ���ǣ�������ط�Ӧ����ʽ���н���_________________________________��
���ǣ�������ط�Ӧ����ʽ���н���_________________________________�� ��ԭ�����ʵ����֤Cu2+��Fe3+������ǿ���Ľ��ۡ�
��ԭ�����ʵ����֤Cu2+��Fe3+������ǿ���Ľ��ۡ� ___________________________________________��
___________________________________________��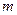 ��
�� ��
�� ��
�� H2O������FeSO4���Ʊ� ����С������ޡ���Ӱ�죬�����ǣ������ӷ���ʽ�ش� ��
H2O������FeSO4���Ʊ� ����С������ޡ���Ӱ�죬�����ǣ������ӷ���ʽ�ش� ��
 CO2�����̼�����ζ��
CO2�����̼�����ζ��