��Ŀ����
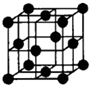
 ��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������B��D��Fԭ��������Ӳ��P�ܼ���������ϵĵ��Ӵ��ڰ���״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ��ͼ��ʾ��E�ĵ縺���ڸ�����������أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ���뵼����Ϻ�GaFΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ�
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������B��D��Fԭ��������Ӳ��P�ܼ���������ϵĵ��Ӵ��ڰ���״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ��ͼ��ʾ��E�ĵ縺���ڸ�����������أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ���뵼����Ϻ�GaFΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ��Իش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��
��1����̬Gaԭ�ӵĺ�������Ų�ʽΪ
��2��A��B��C�ĵ�һ�������ɴ�С��˳��Ϊ
��3��BԪ�صĵ��ʷ�������
��4������A���������������ԭ�Ӳ�ȡ
��5��FH3����BH3
��6������CrE3?6H2O����ˮ�����м��ֲ�ͬ��ɵ������ӣ�ʵ�齫��0.2665gCrE3?6H2O����Һͨ��H-���ӽ�����֬��ֻ�����������ӣ���������������0.125mol/L������������Һ8.00mL�кͣ���֪��������λ��Ϊ6�������������
������A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������B��D��Fԭ��������Ӳ��p�ܼ���������ϵĵ��Ӵ��ڰ���״̬����Χ�����Ų�Ϊns2np3�����ڢ�A�壬��BΪNԪ�أ�DΪPԪ�أ�FΪAsԪ�أ�A��һ�����������Ϊ�Ǽ��Է��ӣ��ɾ����ṹ��֪Ϊ������̼���ӣ���AΪCԪ�أ���C����Ϊ�����ĵ�һ���뵼����ϣ���CΪSi��E�ĵ縺���ڸ�����������ڵ������ڣ���EΪClԪ�أ��ݴ˽��
����⣺A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������B��D��Fԭ��������Ӳ��p�ܼ���������ϵĵ��Ӵ��ڰ���״̬����Χ�����Ų�Ϊns2np3�����ڢ�A�壬��BΪNԪ�أ�DΪPԪ�أ�FΪAsԪ�أ�A��һ�����������Ϊ�Ǽ��Է��ӣ��ɾ����ṹ��֪Ϊ������̼���ӣ���AΪCԪ�أ���C����Ϊ�����ĵ�һ���뵼����ϣ���CΪSi��E�ĵ縺���ڸ�����������ڵ������ڣ���EΪClԪ�أ�
��1��GaΪ31��Ԫ�أ���̬Gaԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p1��
�ʴ�Ϊ��1s22s22p63s23p63d104s24p1��
��2��ͬ����������ҵ�һ�����ܳ��������ƣ�ͬ�������϶��µ�һ�����ܽ��ͣ����һ������ΪN��C��Si��
�ʴ�Ϊ��N��C��Si��
��3�������ĽṹΪN��N����2���м���
�ʴ�Ϊ��2��
��4��A�����������ΪCO2��Ϊֱ�߽ṹ��������Cԭ�Ӳ�ȡsp�ӻ����侧���������������Ϊ���»�����
�ʴ�Ϊ��sp�����»�����
��5��NH3���Ӽ����γ������AsH3���Ӽ䲻���γ����������е���NH3��ȵͣ�
�ʴ�Ϊ���ͣ�NH3���Ӽ����γ������AsH3���Ӽ䲻���γ������
��6��0.2665gCrCl3?6H2O�����ʵ���Ϊ0.2665g��266.5g/mol=0.001mol��������������0.125mol/L������������Һ8.00mL�кͣ���H+�����ʵ���Ϊ0.125mol/L��0.008L=0.001mol�������Եõ�0.001molHCl����1mol������к���1mol���Cl-��1mol�����Ӻ���2mol����Cl-����������λ��Ϊ6����1mol�����ӻ�����4mol����H2O�������������[Cr��H2O��4Cl2]+��
�ʴ�Ϊ��[Cr��H2O��4Cl2]+��
��1��GaΪ31��Ԫ�أ���̬Gaԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s24p1��
�ʴ�Ϊ��1s22s22p63s23p63d104s24p1��
��2��ͬ����������ҵ�һ�����ܳ��������ƣ�ͬ�������϶��µ�һ�����ܽ��ͣ����һ������ΪN��C��Si��
�ʴ�Ϊ��N��C��Si��
��3�������ĽṹΪN��N����2���м���
�ʴ�Ϊ��2��
��4��A�����������ΪCO2��Ϊֱ�߽ṹ��������Cԭ�Ӳ�ȡsp�ӻ����侧���������������Ϊ���»�����
�ʴ�Ϊ��sp�����»�����
��5��NH3���Ӽ����γ������AsH3���Ӽ䲻���γ����������е���NH3��ȵͣ�
�ʴ�Ϊ���ͣ�NH3���Ӽ����γ������AsH3���Ӽ䲻���γ������
��6��0.2665gCrCl3?6H2O�����ʵ���Ϊ0.2665g��266.5g/mol=0.001mol��������������0.125mol/L������������Һ8.00mL�кͣ���H+�����ʵ���Ϊ0.125mol/L��0.008L=0.001mol�������Եõ�0.001molHCl����1mol������к���1mol���Cl-��1mol�����Ӻ���2mol����Cl-����������λ��Ϊ6����1mol�����ӻ�����4mol����H2O�������������[Cr��H2O��4Cl2]+��
�ʴ�Ϊ��[Cr��H2O��4Cl2]+��
���������⿼��λ�ýṹ���ʹ�ϵӦ�ã��������ʽṹ�����ʵ��ۺ���Ŀ���ƶ�Ԫ���ǽ���ؼ���ע���ԭ�ӵĺ�������Ų��ص��Լ�Ԫ�ص���������Ϊͻ�ƿڽ�𣬣�6��Ϊ�״��㡢�ѵ㣬ע��������ӵ����⣬�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
��֪A��B��C��D�ֱ���Cu��Ag��Fe��Al���ֽ����е�һ�֣���֪��A��C������ϡ���ᷴӦ�ų����壻��B��D�������η�Ӧ���û�������D����C��ǿ�Ӧ�ų����壬�ɴ˿����ƶ�A��B��C��D�����ǣ�������
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |
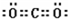
 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�