��Ŀ����
��6�֣����á���ѧ������ʵ���е�Ӧ�á������֪ʶ�������
��1������6.02��1023����ԭ�ӵ�H2SO4�����ʵ�����_________?
��2����4 g NaOH�ܽ���10 mLˮ�У���ϡ�ͳ�1 L������ȡ��10 mL����10 mL��Һ�����ʵ���Ũ��Ϊ_______��
��3����50 mL 0.1 mol��L��1 NaCl��50 mL 0.5 mol��L��1 CaCl2��Һ��Ϻ�����Һ�����Ϊ�������֮�ͣ�������Һ��c (Cl��)Ϊ?______________��
��1������6.02��1023����ԭ�ӵ�H2SO4�����ʵ�����_________?
��2����4 g NaOH�ܽ���10 mLˮ�У���ϡ�ͳ�1 L������ȡ��10 mL����10 mL��Һ�����ʵ���Ũ��Ϊ_______��
��3����50 mL 0.1 mol��L��1 NaCl��50 mL 0.5 mol��L��1 CaCl2��Һ��Ϻ�����Һ�����Ϊ�������֮�ͣ�������Һ��c (Cl��)Ϊ?______________��
��ÿ��2�֣���6�֣���1��0.25mol (2)0.1mol/L (3)0.55mol/L
�����������1������
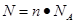 ��֪������6.02��1023����ԭ�����ʵ�����
��֪������6.02��1023����ԭ�����ʵ�����6.02��1023��6.02��1023/mol��1mol�����Ը�������Ļ�ѧʽH2SO4��֪����������ʵ�����1mol��4��0.25mol��
��2������n��m/M��֪��4g�������Ƶ����ʵ�����4g��40g/mol��0.1mol������c��n/V��֪������Һ��Ũ����0.1mol��1L��0.1mol/L��������Һ�Ǿ�һ�ģ�����ȡ����10ml��Һ��Ũ�Ȼ���0.1mol/L��
��3�����ǰ�Ȼ��ƺ��Ȼ����������ӵ����ʵ����ֱ���0.05L��0.1mol/L��0.005mol��0.05L��0.5mol/L��2��0.05mol�����Ի�Ϻ������ӵ����ʵ�����0.005mol��0.05mol��0.055mol������Һ�������100ml�����Ի�Ϻ�������Ũ����0.055mol��0.1L��0.55mol/L��
�������ڽ������ʵ������йؼ���ʱ���ؼ�������Ӧ�ü�����ϵʽ
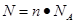 ��n��m/M��
��n��m/M��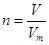 ��
��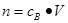 ���ر�Ҫע������Ħ������Ͱ����ӵ����ɵ�ʹ����������ֻ�����������壬��ֻ���ڱ�״���£������Ħ���������22.4L/mol������Ǽ�����Һ������Ũ�ȣ�����Ҫע�����ʵĻ�ѧʽ�Լ���Ӧ�ĵ��뷽��ʽ������ȷ����ij����Ũ�ȡ�����Ǽ������ӵ����ʵ���������Ҫע����Һ������仯��
���ر�Ҫע������Ħ������Ͱ����ӵ����ɵ�ʹ����������ֻ�����������壬��ֻ���ڱ�״���£������Ħ���������22.4L/mol������Ǽ�����Һ������Ũ�ȣ�����Ҫע�����ʵĻ�ѧʽ�Լ���Ӧ�ĵ��뷽��ʽ������ȷ����ij����Ũ�ȡ�����Ǽ������ӵ����ʵ���������Ҫע����Һ������仯��
��ϰ��ϵ�д�
�����Ŀ