��Ŀ����
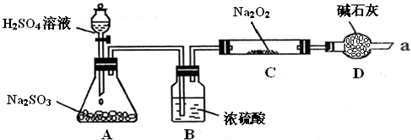
ijͬѧ��ͨ��������ͼ��ʾװ�ã��г�װ������ȥ��ʵ�飬̽��SO2��Na2O2��Ӧ�IJ��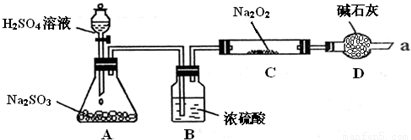
ʵ����÷�ӦǰC�ܵ�����Ϊm1 g����Ӧ��C�ܵ�����Ϊm 2 g����Ӧ��װ��D����������n g���������
��װ��B������______��װ��D������______��
����μ��鷴Ӧ���Ƿ���O2����______��
��C�й������������¼��裺
����1��ֻ��Na2SO3
����2��ֻ��Na2SO4
����3��______��
��1����������2���У���Ӧ����ʽΪ______��
��2����Na2O2��Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣���ͬѧ�������ʵ�飺

�ó����ۣ�������Na2SO4��
�÷����Ƿ����______����ǡ�����������______��
��3��������1������ijͬѧ�Է�Ӧǰ��C�ܵ������ m 2-m 1 ��gΪ�����ƶ�����Na2SO3������Ϊ�� m 2-m 1 ��×126/64g����ʦ��ʾ���㲻����������������е�Ե�ɣ�______��
��4��������2��������μӷ�Ӧ��Na2O2���ʵ���Ϊ______ ���м���ʽ��Ħ����
���𰸡�����������Ũ�������ˮ���Լ��������������Ƶ��������ش�
���ô����ǵ�ľ��������������
��1���������ƺͶ�������Ӧ���������ƣ�
��2��������������ԣ��ܽ������ᱵ����Ϊ��������������ᱵ��
��3�����ݻ�ѧ����ʽ��Ϲ��������IJ����������㣻
��4�����ݹ�������������㣮
����⣺��Ũ��������ˮ�ԣ������ն��������е�ˮ�֣���ֹˮ������������Ʒ�Ӧ�����Ŷ�������ʯ���Ǹ������������ˮ�֣���ֹˮ�����ĸ��ţ���ʯ���Ǽ������ʣ������ն���������������壬��ֹ��Ⱦ�������ʴ�Ϊ������SO2���壬��ֹˮ������Na2O2��Ӧ����ֹ�����е�ˮ�����Ͷ�����̼����Cװ����Na2O2��Ӧ��ͬʱ���չ�����SO2��������Ⱦ������
���ô����ǵ�ľ���ɼ����������ʴ�Ϊ���ô����ǵ�ľ����������ܿ�a���۲����Ƿ�ȼ�գ�
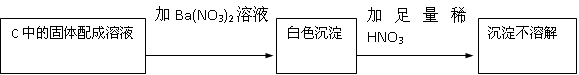
�������������Ƴ�������3���Ǻ����������ƺ������ƵĻ����ʴ�Ϊ����Na2SO3��Na2SO4��
��1���������ƺͶ�������Ӧ���������Ƶ�ԭ������ʽΪ��Na2O2+SO2=Na2SO4���ʴ�Ϊ��Na2O2+SO2=Na2SO4��
��2�����ɵİ�ɫ������������������ᱵ����������Ὣ֮����Ϊ���ᱵ�����ܵó����ۣ�������Na2SO4���ʴ�Ϊ����HNO3�������ԣ��ݴ˲���ȷ��������Na2SO3����Na2SO4������У�
��3��������ݣ�m2-m1�����ڲμӷ�Ӧ��SO2�������ƶ�����Na2SO3������Ϊ��m2-m1��×126/64g�����Ǹ÷�Ӧ������SO2ͬʱ��O2 ���������ԣ� m 2-m 1 �������ڲμӷ�Ӧ��SO2�������ʸ����㲻�������ʴ�Ϊ����Ϊ�÷�Ӧ������SO2ͬʱ��O2 ���������ԣ�m2-m1�������ڲμӷ�Ӧ��SO2�������ʸ����㲻������
��4��������2�������������ƺͶ�������ķ�ӦΪ��Na2O2+SO2=Na2SO4����m2-m1�����ڲμӷ�Ӧ��SO2��������μӷ�Ӧ��Na2O2���ʵ���n��Na2O2��= ��
��
�ʴ�Ϊ��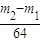 ��
��
���������⿼���˳����������ȡ��ʵ��װ�õ�ѡ����һ���ۺ�֪ʶ��Ŀ���ѶȽϴ�
���ô����ǵ�ľ��������������
��1���������ƺͶ�������Ӧ���������ƣ�
��2��������������ԣ��ܽ������ᱵ����Ϊ��������������ᱵ��
��3�����ݻ�ѧ����ʽ��Ϲ��������IJ����������㣻
��4�����ݹ�������������㣮
����⣺��Ũ��������ˮ�ԣ������ն��������е�ˮ�֣���ֹˮ������������Ʒ�Ӧ�����Ŷ�������ʯ���Ǹ������������ˮ�֣���ֹˮ�����ĸ��ţ���ʯ���Ǽ������ʣ������ն���������������壬��ֹ��Ⱦ�������ʴ�Ϊ������SO2���壬��ֹˮ������Na2O2��Ӧ����ֹ�����е�ˮ�����Ͷ�����̼����Cװ����Na2O2��Ӧ��ͬʱ���չ�����SO2��������Ⱦ������
���ô����ǵ�ľ���ɼ����������ʴ�Ϊ���ô����ǵ�ľ����������ܿ�a���۲����Ƿ�ȼ�գ�
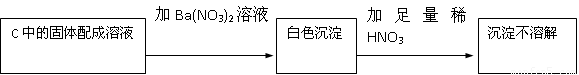
�������������Ƴ�������3���Ǻ����������ƺ������ƵĻ����ʴ�Ϊ����Na2SO3��Na2SO4��
��1���������ƺͶ�������Ӧ���������Ƶ�ԭ������ʽΪ��Na2O2+SO2=Na2SO4���ʴ�Ϊ��Na2O2+SO2=Na2SO4��
��2�����ɵİ�ɫ������������������ᱵ����������Ὣ֮����Ϊ���ᱵ�����ܵó����ۣ�������Na2SO4���ʴ�Ϊ����HNO3�������ԣ��ݴ˲���ȷ��������Na2SO3����Na2SO4������У�
��3��������ݣ�m2-m1�����ڲμӷ�Ӧ��SO2�������ƶ�����Na2SO3������Ϊ��m2-m1��×126/64g�����Ǹ÷�Ӧ������SO2ͬʱ��O2 ���������ԣ� m 2-m 1 �������ڲμӷ�Ӧ��SO2�������ʸ����㲻�������ʴ�Ϊ����Ϊ�÷�Ӧ������SO2ͬʱ��O2 ���������ԣ�m2-m1�������ڲμӷ�Ӧ��SO2�������ʸ����㲻������
��4��������2�������������ƺͶ�������ķ�ӦΪ��Na2O2+SO2=Na2SO4����m2-m1�����ڲμӷ�Ӧ��SO2��������μӷ�Ӧ��Na2O2���ʵ���n��Na2O2��=
 ��
���ʴ�Ϊ��
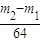 ��
�����������⿼���˳����������ȡ��ʵ��װ�õ�ѡ����һ���ۺ�֪ʶ��Ŀ���ѶȽϴ�

��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
�����Ŀ