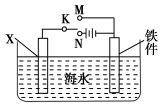
��Ŀ����
����Ŀ��������Ӧ�����ġ��칤������й���������ұ��п�Ĺ��ռ��أ���¯��ʯ����Ҫ�ɷ�Ϊ̼��п��ʮ�װ����һ����ڣ�����Ȼ�������ú̿����ʢ�������н�������Ѻ죬������������ٹ�ȡ��������������ǦҲ����������˵������ȷ����
A. ��ұ��п�ķ�Ӧ�а�����������ԭ��Ӧ
B. ������������ʱʵ����CO��ԭZnO
C. ���˰�п����Ǧ����Ϊп��Ǧ�Ļ�ѧ������ͬ
D. ұ�� Zn ���ܷ�Ӧ����ʽΪ�� 2ZnCO3+C![]() 2Zn+3CO2��
2Zn+3CO2��
���𰸡�C
�������������⣬̼��п��̼��̼�������ģ��ڸ����·�Ӧ��������ұ��п��ԭ�������ܷ����ķ�ӦΪ����ZnCO3![]() ZnO+CO2������CO2+C
ZnO+CO2������CO2+C![]() 2CO����ZnO+CO
2CO����ZnO+CO![]() Zn+CO2������Ϊ̼�������ģ������ܷ�Ӧ�ķ���ʽΪ��ZnCO3+2C
Zn+CO2������Ϊ̼�������ģ������ܷ�Ӧ�ķ���ʽΪ��ZnCO3+2C![]() Zn+3CO����A���Ӧ�ں͢���������ԭ��Ӧ����A��ȷ��B��ɷ�Ӧ�ۿɵã�������������ʱʵ����CO��ԭZnO����B��ȷ��C����칤������أ���������Ǧ�����ͣ�����֮Ի��������˼�ǣ������Ƶõ�п����Ǧ���ֱ�Ǧ�����ʸ����ң���������������Ǧ�����ɴ˿ɼ�п��Ǧ�Ļ�ѧ���ʲ�ͬ����C����D�������ķ�����֪D��ȷ��
Zn+3CO����A���Ӧ�ں͢���������ԭ��Ӧ����A��ȷ��B��ɷ�Ӧ�ۿɵã�������������ʱʵ����CO��ԭZnO����B��ȷ��C����칤������أ���������Ǧ�����ͣ�����֮Ի��������˼�ǣ������Ƶõ�п����Ǧ���ֱ�Ǧ�����ʸ����ң���������������Ǧ�����ɴ˿ɼ�п��Ǧ�Ļ�ѧ���ʲ�ͬ����C����D�������ķ�����֪D��ȷ��

����Ŀ������HCl����״��������ƿ������Ȫʵ���õ���ϡ������Һ���ñ�������������Һ�ζ�����ȷ����ϡ�����ȷ���ʵ���Ũ�ȡ��ش��������⣺
��1���õζ�ʵ��ʢװ��Һ��������_______________������������ʱӦ����__________mL����������������Ϊ50mL����Һ��Ϊ0����������������Һ��ų�����ų�����Һ���_______50mL������������������������������
��2�����õζ�ʵ���÷�̪��ָʾ�����ﵽ�ζ��յ�ʱ����Һ��ɫ��____ɫ��Ϊ____ɫ�ұ���30s�ڲ���ɫ��
��3���������ֲ�ͬŨ�ȵı�����������Һ������Ϊ����ʵ��ǵ�______�֡�
��5.000 mol/L ��0. 5000 mol/L ��0.0500 mol/L
��4���������������ʵı�����������Һ�ζ�ϡ���ᣬ��������������ζ����ʵ���������£�
ʵ���� | ��������������mL�� | ��������������Һ�������mL�� |
1 | 20.00 | 17.30 |
2 | 20.00 | 17.02 |
3 | 20.00 | 16.98 |
���õ�ϡ��������ʵ���Ũ��Ϊ_________________________��
��5�����в������µĽ��������ƫ������ƫС������Ӱ������
�ٵζ���ϴ����ֱ��װ�������������Һ���еζ���___________
�ڵζ�ǰ����ʱ���ӣ��ζ������ʱ���ӣ�___________
�����ú���Na2O���ʵ��������ƹ������Ʊ���Һ��___________
�ܵζ�ǰ����ʽ�ζ��������ݣ��ζ�����ʧ��___________��