��Ŀ����
����Ŀ��25��ʱ���ں�CH3COOH��CH3COO-����Һ�У� CH3COOH��CH3COO-�����и�����ռ�����ʵ�������(a)����ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��

����˵������ȷ����
A. ��pH<4.76����Һ�У�c(CH3COO-)<c(CH3COOH)
B. ��pH=7����Һ�У�a(CH3COOH)=0��a(CH3COO-)=1.0
C. ��pH>4.76����Һ�У�c(CH3COO-)��c(OH-)֮�Ϳɴ���c(H+)
D. ��pH=4.76����Һ�м����ᣬa(CH3COOH)��a(CH3COO-)֮�ͱ��ֲ���
���𰸡�B
��������A. ����ͼ����ж���pH<4.76����Һ�У�c(CH3COO-)<c(CH3COOH)��A��ȷ��B. ����Һ�д��ڴ������ˮ��ƽ���Լ�����ĵ���ƽ�⣬��pH=7����Һ�У����������Ũ�Ȳ�����Ϊ0��B������C. ���ݵ���غ��֪��pH>4.76����Һ�У�c(CH3COO-)��c(OH-)֮�Ϳɴ���c(H+)��C��ȷ��D. ��pH=4.76����Һ�м����ᣬ���������غ��֪a(CH3COOH)��a(CH3COO-)֮�ͱ��ֲ��䣬D��ȷ����ѡB��

����Ŀ��ij�о���ѧϰС��ͬѧ�����ij�п���������������ⶨ���п�����SO2�ĺ������ƶ������о�������
��.���ϱ�����SO2���н�ǿ�Ļ�ԭ�ԣ�����������������Һ������Ӧ��5SO2+2![]() +2H2O===5
+2H2O===5![]() +2Mn2++4H+��
+2Mn2++4H+��
��.���ʵ�鷽��������SO2�Ļ�ԭ��ʹSO2����֪Ũ�ȼ�����ĸ������������Һ��Ӧ��
��.ѡ������ص㣺ij��ҵ����ij����ij����С����ij��ҵ����ij��ͨ��Ŧ��
��.ѡ�����ʱ�䣺���졢���ٽ�Сʱ�����졢���ٽϴ�ʱ����ǰ�����
��.�Բⶨ�Ľ�����г�������������ص�λ������顣
��1����С��ͬѧ������ͼ��ʾװ�ö�������������SO2�ĺ�����
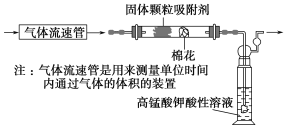
��ͨ�����ǰӦ���е�ʵ�������________________________________��
�ڵ��۲쵽ʵ�������Ϊ________________________________________ʱ��Ӧֹͣͨ������
��ʵ����������������¼��������_________________________________��
��2����ͼ��ʾ��С��ͬѧ����ʵ���õĸ��в�ͬ����������SO2�ĺ�����
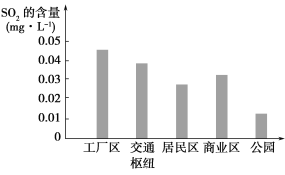
�ٹ���������ͨ��ŦSO2�������Ը�������������ԭ����_____________��
�������йز������һ�����ٹ�����SO2�ŷŵĽ��飺__________________________��
��3���±��Ǹ�С��ͬѧ��õIJ�ͬ��������µij��п�����SO2��ƽ�������������������ٽϴ�ʱSO2ƽ�������ϵ͵�ԭ��
������� | ƽ������(m��s1) | ������SO2��ƽ������(mg��L1) |
��ǰ | 2.0 | 0.03 |
��� | 2.2 | 0.01 |
�� | 23 | 0.015 |
�� | 0.9 | 0.03 |
��_____________________________________________________��
��_____________________________________________________��
����Ŀ�������̿����Ҫ�ɷ�MnO2������SiO2 ��Fe2O3��Al2O3��Cu2(OH)2CO3�ĵ����ʣ�����ϴ������Һ����1mol/LH2SO4��FeSO4��Һ���������������̺����죨Fe2O3��������������

Mn(OH)2 | Fe(OH)2 | Cu(OH)2 | Al(OH)3 | Fe(OH)3 | |
��ʼ����ʱ | 8.3 | 6.3 | 4.7 | 3.4 | 2.7 |
��ȫ����ʱ | 9.8 | 8.3 | 6.7 | 4.4 | 3.2 |
��1�������ʵ����̿�ʹ��ǰ�轫����飬Ŀ����______��
��2������1�к��е���Ҫ������_______��
��3����Һ1�У��Ӱ�ˮ������ҺpH��3.2��ͨ��O2����Ӧ�����ӷ���ʽ��_______������H2O2����O2���Ƿ������������___________��
��4�����̿�������������������Һ��Ӧ�����ӷ���ʽ��_________��
��5����Һ3�м������ܵ����MnS��Ŀ���ǣ������ӷ���ʽ��ʾ��_________��
��6�����ؽᾧ������Һ4�л�ȡ�����̾���IJ����ǣ�__________��