��Ŀ����
��24�֣���Ҫ����գ�
��1�������Ԫ��ԭ�Ӱ뾶��С����������������Ϊ��һ����ɫ�ɳ��صIJ��ϣ��ŵ�ʱ���ǵ�ص����� �����������������±��Ԫ����ԭ�������ĵ�����������������,�ڻ�ѧ��Ӧ���������õ������������ӡ���±��Ԫ���зǽ�������ǿ��Ԫ�����������������ӵĽṹʾ��ͼ�������� ����ÿ��1�֣���6�֣�
��2��Ϊ��ֹ̼�ظֲ˵����⣬ʹ�ú��ر����й��̲˺�Ӧ��ȡ�ļ��״�ʩ�����������������к���������õ�ȼ������������ҪԪ����_ ����������ȼ�պ����ɵĻ�������Ҫ��������������������ÿ��2�֣���6�֣�
��3����Է�������Ϊ58�������ķ���ʽ��________�������ܵĽṹ��ʽ�� _________________��___________________��ÿ��1�֣���3�֣�
��4����6�֣���֪ij��Ӧ�ĸ�����Ũ���������£�
���a= ��b ������
��2S��B�ķ�Ӧ����= ��
��3sĩ������Ӧ��ƽ��ʱ��CŨ��Ϊ0.6 mol/L����A��ת����Ϊ ��
��5����6�֣���ϩͨ����ˮ����ɫ�Ļ�ѧ����ʽ��_____________________����Ӧ���ͣ� __��
���������ɵĻ�ѧ����ʽ��________________________����Ӧ���ͣ�____ ��
17��24�֣�����1��﮸���7,1������
��2���������˵����øɾ��� C H��CO2��H2O
��3��C4H10 ��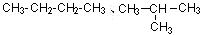
��4����1���� a="3 " b="1 " ��2�֣�����0.1mol/��L��S����60%����2�֣�����6�֣�
��5��CH2 = CH��CH3+ Br��Br ��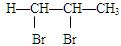 ��1��2��������飩�ӳɷ�Ӧ
��1��2��������飩�ӳɷ�Ӧ ȡ����Ӧ
ȡ����Ӧ
����