��Ŀ����
(12��)ij��ѧ��ȤС����ֻ������������ͭ�Ĺ�ҵ������ȡ�������Ȼ�����Һ���̷�����(FeSO4��7H2O)�͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�
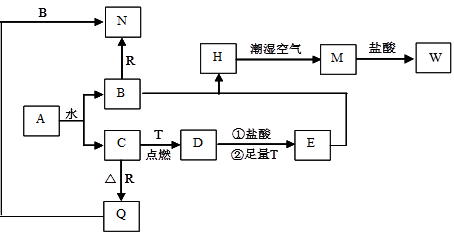
��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ������������������������������������
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�����Ϊ�Ϻ�����;���������ǣ��� �������������� �� ���� ��������
��3����ҺE�������ڿ�����һ��ʱ�����Һ�е������ӳ���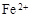 ��
��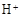 �⣬�����ܴ���������������������Ԫ�ط��ű�ʾ����
�⣬�����ܴ���������������������Ԫ�ط��ű�ʾ����
��4��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ��ǣ�
�������������������������������� ������������ �������� �������� ������������
��5��;���ܷ�����Ӧ�Ļ�ѧ����ʽΪ������������������ �� ������������������
��6��ʵ���Ҵ�CuSO4��Һ��ȡ��������������������Ũ������ȴ�ᾧ������������������Ȼ���

��1��д���Ͻ����ռ���Һ��Ӧ�����ӷ���ʽ������������������������������������
��2������ҺA��AlCl3��Һ��;���Тٺ͢����֣�����Ϊ�Ϻ�����;���������ǣ��� �������������� �� ���� ��������
��3����ҺE�������ڿ�����һ��ʱ�����Һ�е������ӳ���
 ��
��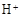 �⣬�����ܴ���������������������Ԫ�ط��ű�ʾ����
�⣬�����ܴ���������������������Ԫ�ط��ű�ʾ������4��������Fͨ������;����ȡ��������;������ȣ�;�������Ծ��е������ŵ��ǣ�
�������������������������������� ������������ �������� �������� ������������
��5��;���ܷ�����Ӧ�Ļ�ѧ����ʽΪ������������������ �� ������������������
��6��ʵ���Ҵ�CuSO4��Һ��ȡ��������������������Ũ������ȴ�ᾧ������������������Ȼ���
(12��)
��1��2Al + 2OH + 2H2O = 2AlO2 + 3H2�� (2��)
��2���ں�����;�����Ƶ�AlCl3��Һ���ȸߣ�����ȡ��AlCl3��Һ�л���NaCl���ʣ�2�֣�
��3��Fe3+��2�֣�
��4���ɱ��ͣ��������ж����壨�������֣�����1�֣�
��5��2Cu+2H2SO4+O2===2CuSO4+2H2O��2�֣�
��6������ϴ�ӣ�2�֣�
��1��2Al + 2OH + 2H2O = 2AlO2 + 3H2�� (2��)
��2���ں�����;�����Ƶ�AlCl3��Һ���ȸߣ�����ȡ��AlCl3��Һ�л���NaCl���ʣ�2�֣�
��3��Fe3+��2�֣�
��4���ɱ��ͣ��������ж����壨�������֣�����1�֣�
��5��2Cu+2H2SO4+O2===2CuSO4+2H2O��2�֣�
��6������ϴ�ӣ�2�֣�
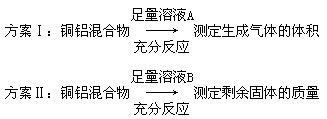
�����������1�������֪�����ռӦ������Ӧ����Һ�У���Һͨ����Ӧ�������ɵIJ�����AlCl3,���ԺϽ������ռӦ������ӦΪ����
��2��;���������ɵ�NaCl���ѳ�ȥ��;����ͨ���������ܽ�ķ�ʽ�ɵõ����Ƚϸߵ�AlCl3��
��3��������ҺE�к���
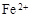 ��
��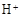 ��Fe2+�ڿ����б�¶һ��ʱ���ױ�����ΪFe3+
��Fe2+�ڿ����б�¶һ��ʱ���ױ�����ΪFe3+��4������;����ʹ��Ũ���ᷢ��������ԭ��ӦCu+2H2SO4��Ũ��=CuSO4+SO2��+2H2O;��Σ�Ũ����ļ۸����ϡ���ᣬ����߳ɱ�
��5��������Cu��ϡ����Ļ���������������������ԭ��Ӧ���䷴Ӧʵ���Ǽ��Խ�������������ķ�Ӧ
��6������Һ����ȡ���ʣ���������Ϊ����Ũ������ȴ�ᾧ������ϴ������Ȼ���

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ