��Ŀ����
���й��ڵ������Һ�����ӹ�ϵ˵���д�����ǣ� ��
| A��pH��ȵ�CH3COONa��NaOH��Na2CO3������Һ�� c(NaOH) < c(Na2CO3) < c(CH3COONa) |
| B��pH = 2��HA��Һ��pH = 12��MOH��Һ����Ȼ�ϣ� c(H��) + c(M��) = c(OH��) + c(A��) |
| C����pH=13��Na2S��Һ�У�Al3+��Cl����CH3COO�����ܴ������� |
| D����ˮ�������������Ũ��Ϊ10-1mol/L����Һ�У�SO32����NO3����Cl����Na�����ܴ������� |
D
��

��ϰ��ϵ�д�
�����Ŀ
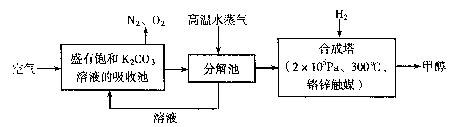
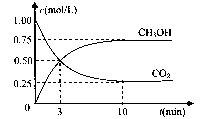

 ����c��H+��+ c��CH3COOH��
����c��H+��+ c��CH3COOH��